Abstract
The dissolution properties of 2-oxo-1,3,5-trinitro-1,3,5-triazacyclohexane (Keto-RDX) in dimethyl sulfoxide (DMSO) were studied by using a RD496-2000 Calvet microcalorimeter at four different temperatures under atmospheric pressure. The heat effects, molar enthalpies (Δ diss H), and differential enthalpies (Δ dif H) of dissolution were determined. Furthermore, the corresponding kinetic equations describing the dissolution processes at the four experimental temperatures are dα/dt = 10−2.43 (1 − α)0.63 (T = 298.15 K), dα/dt = 10−2.41 (1 − α)0.67 (T = 303.15 K), dα/dt = 10−2.40 (1 − α)0.75 (T = 308.15 K), and dα/dt = 10−2.38 (1 − α)0.76 (T = 313.15 K). The determined values of the activation energy E and pre-exponential factor A for the dissolution process are 5.99 kJ mol−1 and 10−1.38 s−1, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The propellant industry is constantly striving for compounds that have high energy and high density characteristics for use in munitions, including both explosives and propellants. Some current high-volume ingredients such as 1,3,5-trinitroperhydro-1,3,5-triazine (RDX) and 1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX) are very effective in providing energy for numerous munitions applications [1,2,3]. The industry, however, continues to seek new compounds having densities near 2.0 g mL−1, detonation pressures greater than 400 kbar, detonation velocities greater than 9000 ms−1, favorable oxygen balance, and good thermal and hydrolytic stability.
One candidate for this type of energetic compound is 2-oxo-1,3,5-trinitro-1,3,5-triazacyclohexane (Keto-RDX or K-6). Keto-RDX is one of the high energy density materials bearing a cyclic dinitro-urea molecule. This compound has a calculated density of near 2.0 g mL−1, a favorable oxygen balance, a calculated detonation velocity of 9270 ms−1, and a calculated detonation pressure of 402 kbar [4,5,6].
Keto-RDX is more energetic and more dense than both RDX and HMX. Keto-RDX has a lower heat of formation but a more favorable oxygen balance than either of the other two. In addition, differential scanning calorimetry measurements show that Keto-RDX has a sharp exothermal peak at 206 °C [7, 8].
Earlier work has mostly focused on synthesis, thermal stability, detonation properties, sensitivity and its use in propellants [9,10,11,12]. However, the thermochemical properties of its solution at different temperatures have never been reported until now.
In the present study, we investigated the dissolution behaviors for Keto-RDX in dimethyl sulfoxide (DMSO) using a RD496-2000 Calvet microcalorimeter. The molar enthalpies (Δ diss H) and the differential enthalpies (Δ dif H) for Keto-RDX in DMSO at different temperatures were obtained, and the kinetic equations of the dissolution processes were also obtained, which will be useful for purification of Keto-RDX in production and can provide valuable information for its applications in the future.
Experimental
Materials
Keto-RDX used in the experiment was prepared and purified by Xi’an Modern Chemistry Research Institute. Its structure was identified by 1H NMR, IR, and elemental analysis, and its purity was more than 99.5%. IR (KBr, υ cm): 3010, 1441 (C–H), 1760 (C=O), 1610, 1282 (N–NO2), 1240, 1153, 1062 (C–N); 1H NMR (500 MHz, DMSO-d 6) δ: 6.36 (s, 4H, 2 × CH2); Elemental anal. (%), calcd. for C3H6N6O4: C 15.25, N 35.39, H 1.70; found: C 15.23, N 35.21, H 1.69. The structure of Keto-RDX is shown in Scheme 1. The sample was stored under vacuum before the experimental measurements. DMSO (ρ = 1.098 − 1.102 g cm−3) as solvent was of analysis reagent grade, and its purity was greater than 99.5%. Deionized water with an electrical conductivity of 0.8 × 10−4–1.2 × 10−4 S m−1 used in the experiments was obtained by purification two times via a sub-boiling distillation device.
Equipment and conditions
All the measurement experiments were performed on a RD496-2000 Calvet microcalorimeter (Mianyang CP Thermal Analysis Instrument Co., Ltd.), which had a sensitivity of 66.5 μV mW−1 at 298.15 K. The microcalorimeter was calibrated by the Joule effect, and the calibration was repeated after each experiment. The standard molar enthalpy of the dissolution of KCl (spectrum purity) in distilled water measured on a RD496-2000 Calvet microcalorimeter at 298.15 K was 17.234 ± 0.041 kJ mol−1, and the relative error was less than 0.04% compared with the literature value 17.241 ± 0.018 kJ mol−1 [13]. This showed that the device for measuring the enthalpy used in this work was reliable. The heat effects were measured at 298.15, 303.15, 308.15, and 313.15 K.
Results and discussion
Thermochemical behavior
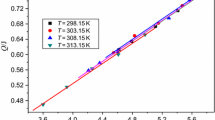
The experimental and calculated values of the heat effects of Keto-RDX dissolved in DMSO at different temperatures are given in Table 1. The molar enthalpies (Δ diss H) for each process are obtained and also listed in Table 1, where a is the amount of the substance, b is the molality of the solution, and Q is the heat effect produced during dissolution. The dissolution is an endothermal process.
In Table 1, the molality of the solution (b) has little influence on the values of the molar enthalpies (Δ diss H) at different temperatures. So the average value of Δ diss H can represent the molar enthalpies of the infinite diluted solution due to the very low molalities of the solution.
The Q versus a relationships of Keto-RDX at different temperatures are shown in Fig. 1. The resulting linear equations for Keto-RDX dissolved in DMSO at different temperatures are shown in Table 2. In Table 2, r is the correlation coefficient. The differential enthalpy (Δ dif H) is the heat effect produced when a molar amount of solute is added to an infinite amount of solution having the same solute already in it [14,15,16].The differential enthalpies (Δ dif H) are obtained from the slope of the equations, and the results are shown in Table 2.
Kinetics of the dissolution processes
Equations (1) and (2) were chosen as the model functions describing the dissolution of Keto-RDX in DMSO [17,18,19].
Substituting α = H/H ∞ into the Eq. 3, we get
In these equations, α is conversion degree, f(α) is the kinetic model function, H represents the enthalpy at time of t, i is any time during the process, H ∞ is the enthalpy of the whole process, k is the dissolution rate of Keto-RDX in DMSO, n is the reaction order, and L is the counting number.
The data needed for this analysis are summarized in Table 3, and four processes are selected at random for each temperature.
Substituting the original data in Table 3, −(dH/dt)i, (H/H ∞)i, H ∞, i = 1,2,…,L, into the kinetic Eq. 4 yields the values of n and lnk that are listed in Table 4.
From Table 4, the values of n and lnk show that the reaction order and the dissolution rate for Keto-RDX in DMSO vary with the experimental temperature, and the values of lnk increase slightly with the experimental temperature increasing.
Substituting the values of n and k in Table 4 into Eq. 3, the kinetic equations describing the dissolution processes of Keto-RDX dissolved in DMSO can be described as
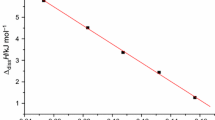
Equation (9) [20,21,22] is applied to calculate the values of the activation energy E and pre-exponential factor A by the slope and the intercept of the linear equation
where A is the pre-exponential factor, E is the activation energy, R is the gas constant, and T is the temperature.
The resulting values of E and A for the dissolution process are 5.99 kJ mol−1 and 10−1.38 s−1, respectively, and the correlation coefficient is 0.9995. The value of E is very low, showing that the reaction proceeds easily over the temperature range of 298.15–313.15 K. The relationship of lnk versus 1/T for the dissolution of Keto-RDX in DMSO is shown in Fig. 2.
Conclusions
-
1.
The values of heat effects of Keto-RDX dissolved in DMSO were determined at different temperatures. The enthalpies can be regarded as the corresponding enthalpies at infinite dilution because of the very low molalities.
-
2.
The differential enthalpies (Δ dif H) are 3.075, 3.594, 4.214, and 4.739 kJ mol−1, and the values of the molar enthalpies (Δ diss H) are 3.075, 3.594, 4.215, and 4.771 kJ mol−1 at temperatures of 298.15, 303.15, 308.15, and 313.15 K.
-
3.
In the dissolution processes of Keto-RDX in DMSO, the reaction order and the dissolution rate vary with temperature during the experimental measurements, and the kinetic parameters E and A thus obtained are 5.99 kJ mol−1 and 10−1.38 s−1, respectively. The small values of E and A explain from kinetic viewpoint that DMSO is a very good solvent for Keto-RDX.
References
Dagley IJ, Kony M. Properties and impact sensitiveness of cyclic nitroamine explosives containing nitroguanidine groups. J Energ Mater. 1995;13:35–56.
Luis GL, Paz D, Ciller J. On the use of AM1 and PM3 methods on energetic compounds. Propellants Explos Pyrotech. 1993;18:33–40.
Huang DS, Rindone RP. High energy insensitive cyclic nitramines. USP 4937340. 1991.
Kony M, Dagley IJ, Leslie DR. 14N NMR studies on cyclic nitramines correlations of chemical shifts with nitrogen partial atomic charge and π-orbital overlap. J Org Chem. 1994;59:5623–6.
Bracuti AJ. Crystal structure of 2-nitroimino-5-nitrohexahydro-1,3,5-triazine. USP 20100326575. 2010.
Metelkina EL. 2-Nitroguanidine derivatives: X. synthesis and nitration of 4-nitriminotetrahydro-1,3,5-oxadiazine and 2-nitriminoohexahydro-1,3,5-triazine and their substituted derivatives. Russian. J Org Chem. 2007;43:1437–40.
Shokrollahl A, Zali A, Pouretedal HR, Keshavarz MH. Synthesis of Keto-RDX and its characterizations calculation. Chinese. J Energ Mater. 2008;16:44–8.
Sikder N, Bulakh NR, Sikder AK. Synthesis, characterization and thermal studies of 2-oxo-1,3,5-trinitro-1,3,5-triazacyclohexane (Keto-RDX or K-6). J Hazard Mater A. 2003;96:109–19.
Cliff MD, Dagley IJ, Parker RP, Walker G. Synthesis of 2-nitroimino-5-nitrohexahydro-1,3,5-triazine via chloride-assisted nitrolysis of a tertiary amine. Propellants Explos Pyrotech. 1998;23:179–81.
Zhou C, Zhou YS, Huo H, Wang BZ, Li JK, Pan Q, Ren XN. Synthesis and thermal behavior for 2-nitrimino-5-nitrohexahydro-1,3,5-triazine. Chin J Explos Propellants. 2012;35:32–5.
Blas L, Klaumünzer M, Pessina F, Braun S, Spitzer D. Nanostructuring of pure and composite-based K6 formulations with low sensitivities. Propellants Explos Pyrotech. 2015;40:938–44.
Shokrolahi A, Zali A, Mousaviazar A, Keshavarz MH, Hajhashemi H. Preparation of nano-K-6 (Nano-Keto RDX) and determination of its characterization and thermolysis. J Energ Mater. 2011;29:115–26.
Marthada VK. The enthalpy of solution of SRM 1655 (KCl) in H2O. J Res Nat Bur Stand. 1980;85:467–8.
Li N, Zhao FQ, XuanCL Gao HX, Xiao LB, Yao EG, Qu WG, Hu RZ. Thermochemical properties of 2,6-diamino-3,5-dinitropyrazine-1-oxide in dimethyl sulfoxide and N-methyl pyrrolidone. J Therm Anal Calorim. 2017;127:2511–6.
Li N, Zhao FQ, Luo Y, Mo HC, Gao HX, Xiao LB, Yao EG, Hu RZ. Study on curing reaction thermokinetics of azide binder/bispropargyl succinate by microcalorimetry. Propellants Explos Pyrotech. 2015;40(6):808–13.
Li N, Zhao FQ, Luo Y, Gao HX, Yao EG, Zhou ZM, Xiao LB, Hu RZ, Jiang HY. Dissolution thermokinetics of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate in dimethyl sulfoxide. J Therm Anal Calorim. 2015;122:1023–7.
Xiao LB, Luo Y, Zhao FQ, Gao HX, Li N, Chen XL, Wang Y, Hu RZ. Dissolution properties of 4,10-dinitro-2,6,8,12-tetraoxa-4,10-diazaisowutrzitane in N-methyl pyrrolidone. J Therm Anal Calorim. 2016;123:659–63.
Li N, Zhao FQ, Luo Y, Gao HX, Xiao LB, Hu RZ, Ju RH. Dissolution property of N-guanylurea dinitramide in dimethyl sulfoxide and N-methyl pyrrolidone. J Therm Anal Calorim. 2014;112:26–9.
Hu RZ, Zhao FQ, Gao HX, Song JR. Foundation and application of calorimetry. Beijing: Science Press; 2011.
Xiao LB, Zhao FQ, Luo Y, Li N, Gao HX, Xue YQ, Cui ZX, Hu RZ. Thermal behavior and safety of dihydroxylammonium 5,5′-bistetrazole-1-1′-diolate. J Therm Anal Calorim. 2016;123:653–7.
Künzel M, Yan QL, Šelešovský J, Zeman S, Matyáš R. Thermal behavior and decomposition kinetics of ETN and its mixtures with PETN and RDX. J Therm Anal Calorim. 2014;115:289–99.
Hu RZ, Gao SL, Zhao FQ, Zhang TL, Zhang JJ. Thermal analysis kinetics. Beijing: Science Press; 2008.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21573173).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, N., Zhao, FQ., Xuan, CL. et al. Thermochemical properties of 2-oxo-1,3,5-trinitro-1,3,5-triazacyclohexane in dimethyl sulfoxide. J Therm Anal Calorim 131, 3047–3052 (2018). https://doi.org/10.1007/s10973-017-6757-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6757-7