Abstract
The molar heat capacities of the room temperature ionic liquid 1-butyl-3-methylimidazolium hexafluoroborate (BMIPF6) were measured by an adiabatic calorimeter in temperature range from 80 to 390 K. The dependence of the molar heat capacity on temperature is given as a function of the reduced temperature (X) by polynomial equations, C P,m (J K−1 mol−1) = 204.75 + 81.421X − 23.828 X 2 + 12.044X 3 + 2.5442X 4 [X = (T − 132.5)/52.5] for the solid phase (80–185 K), C P,m (J K−1 mol−1) = 368.99 + 2.4199X + 1.0027X 2 + 0.43395X 3 [X = (T − 230)/35] for the glass state (195 − 265 K), and C P,m (J K−1 mol−1) = 415.01 + 21.992X − 0.24656X 2 + 0.57770X 3 [X = (T − 337.5)/52.5] for the liquid phase (285–390 K), respectively. According to the polynomial equations and thermodynamic relationship, the values of thermodynamic function of the BMIPF6 relative to 298.15 K were calculated in temperature range from 80 to 390 K with an interval of 5 K. The glass transition of BMIPF6 was measured to be 190.41 K, the enthalpy and entropy of the glass transition were determined to be ΔH g = 2.853 kJ mol−1 and ΔS g = 14.98 J K−1 mol−1, respectively. The results showed that the milting point of the BMIPF6 is 281.83 K, the enthalpy and entropy of phase transition were calculated to be ΔH m = 20.67 kJ mol−1 and ΔS m = 73.34 J K−1 mol−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Room temperature ionic liquids (RTILs) have emerged as a new kind of media alternative to the conventionally used organic solvents, which are generally volatile, flammable and hazardous chemicals [1–3]. Even though ionic liquids such as [EtNH3][NO3], which has a melting point of 285 K, have been known since 1914 [4]; it is only very recently that the usefulness of these systems as a new kind of media for synthetic electrochemical and catalytic applications are being explored [5–11]. Some of the properties that make the room temperature ionic liquids attractive media for various applications are the wide liquid range, non-volatility (negligible vapor pressure), non-flammable nature, less reactivity, and the ability to dissolve a large variety of organic and inorganic substances including even the polymer materials at high concentration. Many of these properties have made the room temperature ionic liquids a nature-friendly “Green Solvent”. The room temperature ionic liquids that are currently the focus of extensive investigation are generally a substituted imidazolium or a pyridinium salt. Even though the solvent properties of different halogenoaluminate(III) ionic liquids (ILs) were studied as early as in 1986 [12], very little is known about the various properties of the contemporary ionic liquids despite their extensive usage in synthetic applications. We refer, for instance, to that data of heat capacity, standard enthalpy of formation, thermodynamic functions and so on, which are paramount for the design of any technological processes, are even scarcer [13].
As a continuation of our previous investigations of RTILs [14–17], in this study, the molar heat capacities of RTIL, BMIPF6 were measured by an adiabatic calorimeter in temperature range from 80 to 390 K. Based on the measured heat capacity data and thermodynamic relationship, the values of thermodynamic function of the BMIPF6 relative to 298.15 K were calculated. The glass translation of BMIPF6 was observed at 190.41 K, the enthalpy and entropy of the transition were calculated. The melting point of the BMIPF6 is 281.83 K; the enthalpy and entropy of phase transition were calculated.
Experimental
Chemicals
The ethylacetate (AR), acetone (AR), acetonitrile (AR) and sodium hexafluoroborate (AR) were of analytical grade and provided by Tianjing Chemical Agent Factory. 1-methylimidazole was vacuum distilled prior to use. 1-chlorobutane (AR) was purified by standard method.
Synthesis of BMIPF6
BMIPF6 was synthesized through alkylation of 1-butyl-3-methylimidazolium chlorate (BMIC) with HPF6 in water as an inert solvent according to the following reaction scheme
BMIPF6 was prepared according to literature procedures [18–25]. We fitted a 500-ml three-necked round-bottom flask with a water condenser, provided it with a Teflon-coated magnetic bar, and equipped with a gas inlet, through which N2 can be purged in case of necessary. 1-methylimidazole (120 mL) and 1-chlorobutane (153 mL) were added into the reaction vessel with continuous magnetic stirring. The reaction mixture was heated under nitrogen N2 at 353.2 K for 96 h with stirring until two phases were formed. The top phase, containing unreacted starting material, was decanted, and ethylacetate (150 mL) was added with thorough mixing. The ethylacetate was decanted followed by the addition of fresh ethylacetate and this step was repeated twice. After the third decanting of ethylacetate, any remaining ethylacetate was removed by heating to 343 K and stirring on a vacuum line. The product is slightly yellow and may be crystalline at room temperature, depending on the amount of water present in that phase. The product was recrystallized twice from dry acetonitrile and dried under vacuum at 343 K for 12 h to yield the pure crystalline 1-butyl-3-methylimidazolium chlorate (BMIC). HPF6 was dropwise added into a solution of BMIC in water and stirred for 36 h. The two-phase system was separated, and the lower phase (BMIPF6) was extracted thrice with 100 mL of deionized water to remove residual HCl. The final ionic liquid product was dried in vacuo at 80 °C for several hours to yield the resulting BMIPF6. The chemical shifts for 1H-NMR spectrum (DMSO, TMS) of the product appear as follows:
δ = 9.113(s,H2), δ = 7.744(d,H4), δ = 7.693 (d,H5), δ = 4.167 (t,NCH2), δ = 3.836 (s,NCH3), δ = 1.741 (m,NCH2CH2), δ = 1.278 (m,NCH2CH2CH2), δ = 0.933 (t,CH3). The 1H-NMR spectra of the product are the same as those in literature [25].
Heat-capacity measurement
Heat-capacity measurements were carried out in a high-precision automatic adiabatic calorimeter described in detail elsewhere [26, 27]. The principle of the calorimeter is based on the Nernst stepwise heating method. The calorimeter mainly consists of a sample cell, an adiabatic (or inner) shield, a guard (outer) shield, a platinum resistance thermometer, an electric heater, two sets of chromel–copper thermocouples and a high vacuum can. The sample cell was made of gold-plated copper and had an inner volume of 48 cm3. Eight gold-plated copper vanes of 0.2 mm thickness were put into the cell to promote heat distribution between the sample and the cell. The platinum resistance thermometer was inserted into the copper sheath, which was soldered in the middle of the sample cell. The heater wire was wound on the surface of the thermometer. The evacuated can was kept within ca. 1 × 10−3 Pa during the heat-capacity measurements so as to eliminate the heat loss due to gas convection. Liquid nitrogen was used as the cooling medium. One set of chromel–copper thermocouples was used to detect the temperature difference between the sample cell and the inner shield. Likewise, the other set of thermocouples was installed between the inner and outer shields. The temperature difference between them was kept within 0.5 mK during the whole experimental process. The sample cell was heated by the standard discrete heating method. The temperature of the cell was measured by a platinum-resistance thermometer. The thermometer was made by the Instrument Manufactory of Yunnan, China, and calibrated at the National Institute of Metrology in terms of the IPTS-90. The temperature increment in a heating period was 2–4 K, and temperature drift was maintained at about 10−4 K min−1 in equilibrium period. All the data were automatically acquired through a data acquisition/switch unit (Model: 34970A, Aglient, USA) and processed online by a computer.
The sample mass used for the heat capacity measurement was 48.7349 g. To verify the reliability of the adiabatic calorimeter, molar heat capacities for the reference standard material, α-Al2O3, were measured. The deviations of our experimental results from the values reported by NIST [28, 29] were within ± 0.2% in the temperature range of 80–400 K.
Results and discussion
Molar heat capacity of BMIPF6
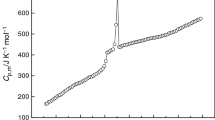
As a continuation of our previous investigations on thermodynamics for new materials significant in science and technology [30–37], the molar heat capacities of the BMIPF6 were determined by using the adiabatic calorimeter in temperature range from 80 to 390 K. The results of the molar heat capacities are listed in Table 1 and shown in Fig. 1. No thermal anomaly was observed or no phase transition took place for solid phase from 80 to 185 K, for glass phase from 195 to 265 K, for liquid phase from 285 to 390 K, respectively.
In order to fit well the heat capacity data to a polynomial equation, the temperature T was replaced by the reduced temperature X which was defined
where T is thermodynamic temperature, T max and T min are the maximum and the minimum of the temperature in the experimental temperature range. Then −1 ≤ X≤1.
The values of molar heat capacities of the BMIPF6 were fitted to the following polynomial expressions by the least square method. For the solid phase (80–185 K):
where reduced temperature, X = (T − 132.5)/52.5. The above equation is valid from 80 to 185 K, with an uncertainty of ±0.3%. The correlation coefficient of the fitted curve, R 2 = 0.9999. For the glass phase (195–265 K):
where reduced temperature X = (T − 230)/35. The above equation is valid from 195 to 265 K with an uncertainty of ±0.4%. The correlation coefficient of the fitted curve, R 2 = 0.9485. For the liquid phase (285–390 K):
where reduced temperature X = (T − 337.5)/52.5. The above equation is valid from 285 to 390 K with an uncertainty of ±0.2%. The correlation coefficient of the fitted curve, R 2 = 0.9999.
Thermodynamic parameters of glass transition
It can be seen from Fig. 1 that the heat capacity jump, corresponding to the glass transition of the BMIPF6, took place in the range from 185 to 195 K. The temperature of the glass transition, T g, was determined to be 190.41 K. The molar enthalpy, ΔH g, and entropy, ΔS g, of the glass transition were derived by the following equation [35]:
where T i is a little lower than T g and T f a little higher than T g, \( \overline{H}_{\text{cell}} \) is the heat capacity of the empty calorimeter, Q is the total energy introduced into the calorimeter during the course of glass transition. The results of the calculation were ΔH g = 2.853 kJ mol−1 and ΔS g = 14.98 J K−1 mol−1.
The molar heat capacity reached maxima in temperature from 265 to 285 K as shown in Fig. 1, the phase transition took place in the temperature range. The phase transition temperature was determined to be T m = 281.83 K which was corresponding to the solid–liquid phase transition of the ionic liquid. Using similar Eqs. 5 and 6, the values of molar enthalpy of fusion, ΔH m, and of molar entropy of fusion, ΔS m, can be calculated. The results were ΔH m = 20.67 kJ mol−1 and ΔS m = 73.34 J K−1 mol−1.
Thermodynamic functions of ionic liquid BMIPF6
Thermodynamic functions of BMIPF6 were calculated based on the empirical Eqs. 2–4, and the relationships of the thermodynamic functions:
The thermodynamic functions relative to the reference temperature (298.15 K) were calculated by Eqs. 7 and 8 in the temperature range from 80 to 390 K with an interval of 5 K and are listed in Table 2.
References
Gordon CM, Holbrey JD, Kennedy AR, Seddon KR. Ionic liquid crystals: hexafluorophosphate salts. J Mater Chem. 1998;8:2627–36.
Fuller J, Cartin RT, Osteryoung RA. The room temperature ionic liquid 1-ethyl-3-methylimidazolium tetrafluoroborate: electrochemical couples and physical properties. J Electrochem Soc. 1997;144:3881–6.
Sun J, Forsyth M, Macfarlane DR. Room-temperature molten salts based on the quaternary ammonium ion. J Phys Chem B. 1998;102:8858–64.
Welton T. Room-temperature ionic liquids. Chem Rev. 1999;99:2071–83.
Carmichael AJ, Seddon KR. Polarity study of some 1-alkyl-3-methylimidazolium ambient-temperature ionic liquids with the solvatochromic dye, Nile Red. J Phys Org Chem. 2000;13:591–5.
Song CE, Shim WH, Roh EJ, Lee SG, Choi JH. Ionic liquids as powerful media in scandium triflate catalysed Diels-Alder reactions: significant rate acceleration, selectivity improvement and easy recycling of catalyst. Chem Commun. 2001;12:1122–3.
Wasserscheid P, Gordon CM, Hilgers C, Muldoon MJ, Dunkin IR. Ionic liquids: polar, but weakly coordinating solvents for the first biphasic oligomerisation of ethene to higher alpha-olefins with cationic Ni complexes. Chem Commun. 2001;13:1186–7.
Wheeler C, West KN, Liotta CL, Eckert CA. Ionic liquids as catalytic green solvents for nucleophilic displacement reactions. Chem Commun. 2001;10:887–8.
Endres F. Electrodeposition of a thin germanium film on gold from a room temperature ionic liquid. Phys Chem Chem Phys. 2001;3:3165–74.
Najdanovic-Visak V, Esperanca MSS J, Rebelo LPN. Phase behaviour of room temperature ionic liquid solutions: an unusually large co-solvent effect in (water plus ethanol). Phys Chem Chem Phys. 2002;4:1701–3.
Vasserscheid P, Keim W. Ionic liquids—new “solutions” for transition metal catalysis. Angew Chem Int Ed. 2000;39:3772–89.
Appleby D, Hussey CL, Seddon KR, Turp JE. Room-temperature ionic liquids as solvents for electronic absorption-spectroscopy of halide-complexes. Nature. 1986;32:3614–6.
Anthony JL, Maginn FJ, Brennecke. Solution thermodynamics of imidazolium-based ionic liquids and water. J Phys Chem B. 2001;105:10942–9.
Yang JZ, Tian P, He LL, Xu WG. Studies on room temperature ionic liquid InCl3-EMIC. Fluid Phase Equilib. 2003;204:295–302.
Yang JZ, Xu WG, Zhang QG. Thermodynamics of {1-methyl-3-butylimidazolium chloride plus iron(III) chloride}. J Chem Thermodyn. 2003;35:1855–60.
Yang JZ, Zhang ZH, Fang DW, Li JG, Guan W, Tong J. Studies on enthalpy of solution for ionic liquid: the system of 1-methyl-3-ethylimidazolium tetrafluoroborate (EMIBF4). Fluid Phase Equilib. 2006;247:80–3.
Yang JZ, Tian P, Xu WG. Studies on an ionic liquid prepared from InCl3 and 1-methyl-3-butylimidazolium chloride. Thermochim Acta. 2004;4:121–5.
Holbrey JD, Reichert WM, Swatloski RP. Efficient, halide free synthesis of new, low cost ionic liquids: 1,3-dialkylimidazolium salts containing methyl- and ethyl-sulfate anions. Green Chem. 2002;4:407–13.
Fuller J, Osteryoung RA, Carlin RT. Rechargeable lithium and sodium anodes in chloroaluminate molten-salts containing thionyl chloride. J Electrochem Soc. 1995;142:3632–36.
Jacobsen EN, Marko I, Sharpless KB. Asymmetric dihydroxylation via ligand-accelerated catalysis. J Am Chem Soc. 1988;110:1968–70.
Bonhote P, Dias AP, Papageorgiou N, Kalyanasundaram K, Gratzel M. Hydrophobic, highly conductive ambient-temperature molten salts. Inorg Chem. 1996;35:1168–78.
Suarez PAZ, Dullius JEL, Einloft S, Souza RFD, Dupnot J. The use of new ionic liquids in two-phase catalytic hydrogenation reaction by rhodium complexes. Polyhedron. 1996;157:1217–9.
Dyson PJ, Grossel MC, Srinivasan N, Vine T, Welton T, Williams DJ, et al. Organometallic synthesis in ambient temperature chloroaluminate(III) ionic liquids. Ligand exchange reactions of ferrocene. J Chem Soc Dalton Trans. 1997;19:3465–9.
Lau RM, Van Rantwijk F, Seddon KR, Sheldon RA. Lipase-catalyzed reactions in ionic liquids. Org Lett. 2000;2:4189–91.
Jiang JD, Gao S, Li ZH, Su GY. Gel polymer electrolytes prepared by in situ polymerization of vinyl monomers in room-temperature ionic liquids. React Funct Polym. 2006;66:1141–8.
Tan ZC, Sun GY, Sun Y, Yin AX, Wang WB, Ye JC, et al. An adiabatic low-temperature calorimeter for heat-capacity measurement of small samples. J Therm Anal Calorim. 1995;45:59–67.
Tan ZC, Sun LX, Meng Sh, Li L, Xu F, Liu BP. Heat capacities and thermodynamic functions of p-chlorobenzoic acid. J Chem Thermodyn. 2002;34:1417–29.
Archer DG. Thermodynamic properties of synthetic sapphire (alpha-al2o3), standard reference material 720 and the effect of temperature-scale differences on thermodynamic properties. J Phys Chem Ref Data. 1993;22:1441–53.
Qi YN, Xu F, Ma HJ, Sun LX, Zhang J, Jiang T. Thermal stability and glass transition behavior of PANI/gamma-Al2O3 composites. J Therm Anal Calorim. 2008;91:219–23.
Wang SX, Tan ZC, Di YY, Xu F, Wang MH, Sun LX, et al. Calorimetric study and thermal analysis of crystalline nicotinic acid. J Therm Anal Calorim. 2004;76:335–42.
Xu F, Sun LX, Tan ZC, Liang JG. Low-temperature heat capacities and standard molar enthalpy of formation of aspirin. J Therm Anal Calorim. 2004;76:481–9.
Nan ZD, Tan ZC. Low-temperature heat capacities and derived thermodynamic functions of cyclohexane. J Therm Anal Calorim. 2004;76:955–63.
Xue B, Wang JY, Tan ZC, Lu SW, Meng SH. Heat capacities and thermodynamic properties of chrysanthemic acid. J Therm Anal Calorim. 2004;76:965–73.
Song YJ, Tan ZC, Lu SW, Xue Y. Thermochemical and thermal analysis on N-(p-methylphenyl)-N′-(2-pyridyl)urea. J Therm Anal Calorim. 2004;77:873–82.
Tan ZC, Xue B, Lu SW, Meng SH, Yuan XH, Song YJ. Heat capacities and thermodynamic properties of fenpropathrin (C22H23O3N). J Therm Anal Calorim. 2001;63:297–308.
Qiu SJ, Chu HL, Zhang J, Qi YN, Sun LX, Xu F. Heat capacities and thermodynamic properties of CoPc and CoTMPP. J Therm Anal Calorim. 2008;91:841–8.
Zhang J, Zeng JL, Liu YY, Sun LX, Xu F, You WS, et al. Thermal decomposition kinetics of the synthetic complex Pb(1, 4-BDC)·(DMF)(H2O). J Therm Anal Calorim. 2008;91:189–93.
Acknowledgements
The authors gratefully acknowledge the financial support for this work from the National Natural Science Foundation of China (No. 2083309, 20873148, 20903095,50671098 and U0734005), 863 projects (2007AA05Z115 and 2007AA05Z102), the National Basic Research Program (973 program) of China (2010CB631303) and IUPAC (Project No. 2008-006-3-100).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, Z.H., Cui, T., Zhang, J.L. et al. Thermodynamic investigation of room temperature ionic liquid. J Therm Anal Calorim 101, 1143–1148 (2010). https://doi.org/10.1007/s10973-009-0610-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0610-6