Abstract
The synthesis for a series of ferrite (MIIFe2O4) and cobaltite (MIICo2O4) spinels was investigated where MII is Mg, Co, Ni, Cu or Zn. The ferrites were prepared at a calcination temperature of 800 °C; the cobaltites at 500 °C. TG–MS indicated that reduction of CoIII to CoII occurs at ca. 800 °C, hence, the lower calcination temperature. For both the ferrites and the cobaltites, the evolution of water and CO2 during the calcination suggests the presence of both species in the precipitates. The observed mass losses indicated that the precursor basic carbonate precipitates for the cobaltite synthesis were predominantly carbonate, while the precursor basic carbonate precipitates for ferrite synthesis were predominantly hydroxide in character. XRD data showed successful synthesis of the ferrites with minimal contamination from the parent oxides, while the cobaltites were observed to be predominantly of the spinel structure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Spinels are an important class of material due to their catalytic and magnetic properties. Ferrites are manufactured for their magnetic properties, in particular, for their high frequency response in high frequency transformer cores, and magnetic memories and for their catalytic activity [1, 2]. They have also been found to be useful anticorrosive pigments for ceramic applications [3]. Cobaltites also show significant catalytic activity [4–6]. These spinels have also been developed as the basis for a number of undergraduate laboratory experiments, such as the synthesis of lithium manganite cathode materials [5], copper ferrite catalysts [7] and magnetite ferrofluids [8]. Spinels are also used as a basis for the conceptual development and application of crystal field theory, particularly, through the calculation of site preference energies in determination of inverse or normal spinel structures [8, 9] which has also been demonstrated through the use of ping-pong ball models to aid the understanding of structure development [9, 10].
Various methods for the synthesis of spinels have been studied, such as co-precipitation of the hydroxides [11] and oxylates [12], complexometric (sol–gel) synthesis through glycolate [13], ethylenediaminetetraacetate (EDTA) [14], fumarate [15, 16] and citrate intermediates [17, 18], organometallic synthesis in a polymer matrices [19], mechanochemical methods using binary oxides [20] and synthesis within silica ampoules [21, 22]. This article investigates a simple co-precipitation method using sulphate precursor solutions precipitated with sodium carbonate/ammonia solution followed by calcination at elevated temperature. The calcination process was characterised using thermogravimetric analysis coupled with mass spectrometry (TG–MS) for evolved gas analysis; characterisation of the calcination products was carried out using X-ray diffraction (XRD).
Experimental
Materials
All chemicals used in the preparation of the spinels were analytical ACS reagents supplied by Sigma-Aldrich; CuSO4·5H2O, CoSO4·7H2O, (NH4)2Fe(SO4)2·6H2O, MgSO4, NiSO4(H2O)6 and ZnSO4(H2O)7, Na2CO3 and saturated ammonia solution.
Procedure
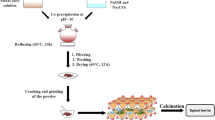
Precursor solutions in the required stoichiometric ratios were prepared by mixing 100 mL of 0.2 M solutions of ammonium iron (II) sulphate (for ferrite synthesis) or cobalt (II) sulphate (for cobaltite synthesis) with 100 mL of 0.1 M solutions of MIISO4, where MII = Cu, Co, Mg, Ni or Zn. Basic carbonates were then precipitated by adding a 1 M sodium carbonate solution/0.59 M ammonia solution, with stirring, in 5 mL aliquots until a pH of 8 was reached (ca. 30–40 mL). The resulting precipitate was then recovered by vacuum filtration and dried in an air oven at 110 °C overnight.
Before calcination, each mixed, dried basic carbonate is ground to a fine powder using a mortar and pestle, and approximately 100 mg is reserved for thermal analysis. The mixed basic carbonates are then calcined at elevated temperature for 1 h: 500 °C for the cobaltites and 800 °C for the ferrites.
Composition and phase analysis
XRD patterns were obtained over a range of 5°–90° 2θ at a step of 0.02° with 2 s accumulation per step, using Siemens D5000 Diffractometer, with a Cu-Kα radiation source. Phase identification was carried out using ICDD (International Committee for Diffraction Data) JCPDS (Joint Committee for Powder Diffraction Standards) database to match the JCPDS Powder Diffraction File (PDF) card data.
The samples were examined by TG–MS using a Setaram Setsys 16/18 Thermobalance coupled with a Balzers ThermoStar mass spectrometer for evolved gas analysis (EGA). Experiments were carried out by placing circa 35 mg of the mixed carbonate sample into a 100 μL platinum crucible and heating at a rate of 10 °C/min from ambient temperature to 1000 °C in an air atmosphere (instrument grade air supplied by BOC Australia) at a flow rate of 20 mL/min.
The peaks in the mass spectra chosen for analysis were at 14, 15, 18, 28, 44, 46 and 64 amu with an aim to identify the presence of water (H2O+), carbon dioxide (C+ and CO2 +), sulphur dioxide (SO2 +) and ammonia (NH+) as decomposition products. As only water and carbon dioxide were detected using EGA, only the data for these mass species are reported.
Results
TG–MS data for the series of ferrites is shown in Fig. 1 and for the cobaltites in Fig. 2. The TG data suggest that the calcination process for the cobaltites is complete by 500 °C; hence, this was the temperature selected for the calcination of the cobaltites. This low temperature is also prescribed by the necessity to calcine at a temperature where the oxidised CoIII state is present as at higher temperatures thermal reduction to the MII state occurs. This is observed for the cobaltites between 800 and 900 °C. The ferrites are observed to undergo mass loss up to temperatures above 600 °C; in each case, calcination appears to be complete by 800 °C; hence, this was the temperature selected for the calcination of the ferrites.
EGA suggests that, for both the ferrites and the cobaltites, the precursor precipitates contain significant proportions of hydroxide and carbonate. Mass losses listed in Table 1 confirm the presence of both carbonate and hydroxide species suggesting that the decomposition of the precipitate precursors is of the form:
With the exception of the zinc cobaltite, the cobaltites are predominantly carbonate; the ferrites, on the other hand, are predominantly hydroxide. It was not possible to identify which of these species were present in the precursor precipitates from XRD, as the patterns produced only very broad diffuse peaks (data not shown) which did not correlate well with carbonate or hydroxide species of corresponding metal ions present. The X-ray amorphous character of the basic carbonate precipitate suggests that the precipitate is finely divided and, at best, microcrystalline.
The basic carbonate decomposition step is accompanied by the oxidation of FeII to FeIII and CoII to CoIII to produce the spinel structure which is confirmed by the XRD data for ferrites calcined at 800 °C shown in Fig. 3 and the cobaltites calcined at 500 °C in Fig. 4. The XRD patterns for the phases present correlate well with the spinel structures based on JCPDS PDF card data. The ferrites produced products with a high degree of crystallinity with minimal presence of contaminant phases. The cobaltites produced predominantly cobaltite spinels as the main product, but in each case, a trace of the MII oxide is present (ZnO, NiO, MgO and CuO). It is possible that the reaction has not reached completion which may be addressed by either raising the temperature or increasing the calcination time, although the formation of cobalt oxide spinel (CoIICo III2 O4) may also be responsible for the incomplete reaction.
Conclusions
The article reports a simple synthesis of ferrite and cobaltite spinels through the co-precipitation method. Spinels are the main products of the reaction although, for the cobaltites, the individual oxide phases were also observed. TG–MS allowed the decomposition reaction to be monitored and provided the basis for determining the calcination temperatures of the basic carbonates; 500 °C for the cobaltites and 800 °C for the ferrites. The calcination temperature for the ferrites appears to be optimal. Mixed oxide phases present along with the cobaltite spinels suggest that some further optimisation of the cobaltite calcination temperature may be achieved.
References
Hyeon T. Chemical synthesis of magnetic nanoparticles. Chem Commun. 2003;(8):927–34.
Hirai P, Sengupta S. Spinel ferrites as catalysts: a study on catalytic effect of coprecipitated ferrites on hydrogen peroxide decomposition. Can J Chem. 1991;69:33–6.
Konvička T, Mošner P, Šolc Z. Investigation of the non-isothermal kinetics of the formation of ZnFe2O4 and ZnCr2O4. J Therm Anal Calorim. 2000;60(2):629–40.
Paike VV, et al. Synthesis of spinel CoFe2O4 Via the co-precipitation method using tetraalkyl ammonium hydroxides as precipitating agents. J Am Ceram Soc. 2007;90(9):3009–12.
Klissurski DG, Uzunova EL. Synthesis of high-dispersity zinc cobaltite from coprecipitated hydroxycarbonate precursor. J Mater Sci Lett. 1990;9(5):576–9.
Klissurski DG, Uzunova EL. Synthesis of nickel cobaltite spinel from coprecipitated nickel-cobalt hydroxide carbonate. Chem Mater. 1991;3:1060–3.
Zümreoglu-Karan B, Yilmazer E. Preparation of spinel-type cathode materials from carbonate/oxalate powder mixtures. A solid-state experiment for advanced undergraduate inorganic chemistry laboratory. J Chem Educ. 2000;77(9):120.
Berger P, et al. Preparation and properties of an aqueous ferrofluid. J Chem Educ. 1999;76(7):943.
Suchow L. A detailed, simple crystal field consideration of the normal spinel structure of Co3O4. J Chem Educ. 1976;53(9):560.
Weller MT. Inorganic materials chemistry. Oxford: Oxford University Press; 1994.
Szczygiel I, Winiarska K. Low-temperature synthesis and characterization of the Mn–Zn ferrite. J Therm Anal Calorim. 2011;104:557–83.
Bordeneuve H, Rousset A, Tenailleau C, Guillemet-Fritsch S. Cation distribution in manganese cobaltite spinels Co3-xMnxO4 (0 ≤ x ≤ 1) determined by thermal analysis. J Therm Anal Calorim. 2010;101:137–42.
Pacurariu C, et al. New synthesis methods of MgAl2O4 spinel. J Eur Ceram Soc. 2007;27(2–3):707–10.
Thang PD, Rijnders G, Blank DHA. Spinel cobalt ferrite by complexometric synthesis. J Magnet Magnet Mater. 2005;295(3):251–6.
Gawas UB, Verenkar V/M/S, Mojumdar SC. Synthesis and characterisation of Ni0.6Ni0.4Fe2O4 nano-particles obtained by autocatalytic thermal decomposition of carboxylato-hydrazinate complex. J Therm Anal Calorim. 2011;104:879–83.
Gonsalves LR, Mojumdar SC, Verenkar VMS. Synthesis of cobalt nickel ferrite nanoparticles via autocatalytic decomposition of the precursor. J Therm Anal Calorim. 2010;100:789–92.
Waqas H, Qureshi AH. Influence of pH on nanosized Mn–Zn ferrite synthesized by sol–gel auto combustion process. J Therm Anal Calorim. 2009;98:355–60.
Waqas H, Qureshi AH. Low temperature sintering study of nanosized Mn–Zn ferrites synthesized by sol–gel auto combustion process. J Therm Anal Calorim. 2010;100:529–35.
Sen A, Pramanik P. Preparation of nano-sized calcium, magnesium & zinc chromite powder through metalo-organic precursor solutions. J Mater Synth Process 2002;10(3):107–11.
Šepelák V, Heitjans P, Becker K. Nanoscale spinel ferrites prepared by mechanochemical route. J Therm Anal Calorim. 2007;90(1):93–7.
Turkin AI, Drebushchak VA. Synthesis and calorimetric investigation of stoichiometric Fe-spinels: MgFe2O4. J Cryst Growth. 2004;265(1–2):165–7.
Turkin AI, et al. Low temperature heat capacity of magnesioferrite, MgFe2O4. J Therm Anal Calorim. 2008;92(3):717–21.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Angus, K., Thomas, P. & Guerbois, JP. Synthesis and characterisation of cobaltite and ferrite spinels using thermogravimetric analysis and X-ray crystallography. J Therm Anal Calorim 108, 449–452 (2012). https://doi.org/10.1007/s10973-011-1863-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1863-4