Abstract
Two aluminate spinel materials (ZnAl2O4 and NiAl2O4) were synthesized by the citrate precursor method. The citrate precursors consisting of coprecipitated citrates of Zn2+ or Ni2+ and aluminum were first subjected to thermal analysis (TG-DSC) for determining the optimum temperature for annealing. Two step decomposition was observed incorporating dehydration and formation of the aluminate. The second step gives an endo peak (−2937 J/g) at 356 °C in the DSC curve of the coprecipitated nickel(II) citrate–aluminum citrate gel in O2 atmosphere. Kinetic/mechanistic analysis of the TG data has also been carried out and values of E a, ΔS #, ΔG #, and A were approximated. On the basis of the findings, 450 °C has been chosen for annealing of the gels. Annealing has also been done at 650 °C for 1 h in muffle furnace in an attempt to obtain nanometric particles of aluminates (MAl2O4) {M = Ni, Zn} and to find out their magnetic properties which could render them useful for chemical sensing applications, etc. The TG-DSC curves of various powders which were obtained on annealing at the two temperatures did exhibit thermal instability when carried out in N2 atmosphere. NiAl2O4 and ZnAl2O4 spinels (particle size 17 and 34 nm, respectively) are obtained in pure crystalline phase at 650 °C. ZnAl2O4 prepared this way shows coercivity values of 470 and 58.37 G and NiAl2O4, 107 and 23.24 G when annealed at 450 and 650 °C, respectively. ZnAl2O4 prepared by a polymer precursor method and annealed at 1000 °C, has earlier been reported to have coercivity value of 469 G. Thus, the citrate precursor method is good for the synthesis of ZnAl2O4, producing single phase nanocrystalline powder of high quality and crystallinity. The value of magnetization was found to be small in the present case for the NiAl2O4 spinel obtained at 450 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aluminate content brings diverse applications to materials. Ceramic materials (cements, castable ceramics, bioceramics, and electroceramics) are normally formed from cubic crystal systems [1] which include garnets and spinels. Many rare earth aluminate garnets are used as laser host when doped with Nd(III) or Yb(III) and also as scintillators and phosphors. Upon doping, interesting properties have been observed. Eu2+, R3+-doping for example, brings thermoluminiscence properties to calcium aluminate materials [2]. And BaMgAl10O17:Eu2+ (BAM), is an important blue phosphor of plasma display panel (PDP) [3]. The rare earth aluminate glasses find use as alternatives to sapphire for use in infrared windows. Single crystals of lanthanum aluminate are known to have application as a substrate for deposition of thin films of the high temperature superconductors such as YBa2Cu3O7 [4]. High-temperature electrical property measurements (electrical conductivity, thermoelectric coefficient) on polycrystalline CuAlO2 exhibit characteristic small polaron features [5]. Transition metal-aluminum mixed oxide catalysts (Cu, Ni, and Co–Al2O3), with spinel-type structure, show high activity and selectivity for the selective reduction of NO by C3H6 in excess oxygen even in the presence of 10% water [6]. These were prepared by coprecipitation method.
Being basically refractory materials, the synthesis of aluminates usually involves solid state growth of mixture of pure oxides. Presence of other oxides may stimulate (e.g., copper oxides) or hinder (e.g., Li2O) crystallization of alumina [7]. Among alternative synthetic routes is the sol–gel method that has significant advantage of being able to bring desired porosity in the materials. An important parameter is the ligand used if complexes have been used for such syntheses. As in spinel ferrites [8], the mean crystallite size has been found to be depending on the ligand used in the sol–gel precursor method used for preparing the Mg–Al spinels [9]. ZnAl2O4 and Sn–ZnAl2O4 have been synthesized by coprecipitation, sol–gel, and impregnation methods [10]. Solutions of nickel and aluminum nitrates have been mixed to furnish hydroxide samples of different compositions by co-precipitation using dilute NH4OH. Impregnation of hydrated Al2O3, preheated at 600 and 900 °C, has been done with nickel nitrate solution in an equimolar ratio [11]. In such case, the degree of crystallinity of the spinel has been found to be depending on the calcinations temperature. Doping is also done by wet impregnation method. Wet impregnation of finely powdered Al(OH)3 with Zn(NO3)2 and Cu(NO3)2 solutions has been reported to be successfully carried out by treating the doped solids with LiNO3 solution before treatment with zinc and copper nitrate solutions to get Li2O-doped CuO–ZnO/Al2O3 mixed solids [12]. Thermal evolution of Na(Li) polyaluminate microspheres have also been produced by sol–gel method [13]. Zinc aluminate spinels have been reported to be forming at 800 °C when the mixed nitrate salts were used for the formation of zinc basic carbonate and aluminum hydroxide as the precursor to a 2ZnO–3Al2O3 spinel system [14]. The unit cells of the ZnAl2O4, NiAl2O4, and CoAl2O4 are capable of holding a large number of divalent and trivalent cations in solid solution so they are interesting.
Citrate precursor methods and polymeric precursor methods have recently been used for obtaining some other interesting aluminates in general and nanoparticles in particular [15–18]. In the present study, thermal analysis (TG-DSC) of coprecipitated aluminum(III) citrate–nickel(II) citrate gel has been carried out in O2 atmosphere. The citrate precursors consisting of coprecipitated citrates of Zn2+ or Ni2+ and aluminum were first subjected to thermal analysis (TG-DSC) for determining the optimum temperature for annealing. In both, the two steps of decomposition incorporate dehydration and formation of the aluminate. Thermogravimetric data and their kinetic/mechanistic data provide insight into the thermodynamics, kinetics, and thermal stability of the substances [19–28]. With this view, kinetic/mechanistic analysis of the TG data has also been carried out for the step corresponding to the endo peak and values of E a, ΔS #, ΔG #, and A were approximated. On the basis of the findings, 450 °C has been chosen for annealing of the gels. Annealing has been done at 650 °C as well for 1 h in muffle furnace in an attempt to obtain nanometric particles of aluminates (MAl2O4) {M = Ni, Zn} and to find out their magnetic properties which could render them useful for chemical sensing applications, etc. This way ZnAl2O4 and NiAl2O4 spinels have been prepared. Characterization of the annealed powder was done using X-ray diffraction for phase and materials crystalline size and Vibrating Sample Magnetometer for magnetic parameters at room temperature.
Experimental
Materials
Zinc nitrate, nickel nitrate, and aluminum nitrate (all AR) were taken in the required stoichiometric proportions as starting materials. Aqueous solutions of these salts were prepared separately (equimolar ratio) by dissolving the respective salts in minimal amount of deionized water while stirring constantly. After mixing the respective pairs of solutions, an aqueous solution of citric acid was added to it which was prepared in adequate quantity by weight (equimolar ratio). The mixture was heated around 60–80 °C for 2 h with continuous stirring. This solution was allowed to cool at room temperature and subsequently dried at 60–65 °C in an oven until it formed a fluffy mass.
Thermal analysis and kinetic analysis
The coprecipitated citrate gels of Zn2+ or Ni2+, and aluminum thus obtained were first subjected to thermal analysis (TG-DSC) to get an idea of a minimum temperature for annealing. Its thermogravimetric analysis was carried out in oxygen at heating rate of 10 °C/min. The citrate gel sample was dehydrated by heating up to 200 °C and the measurement was carried out without removing the sample from the instrument up to the desired temperature range using O2 gas (Fig. 1).
The decomposition is over around 430 °C. Kinetic/mechanistic analysis of the TG data has been carried out for the endo step and values of E a, ΔS #, ΔG #, and A were approximated for the nickel sample. Calculations were made for the respective f(α) values for different possible models and by constructing spreadsheets on MS Office Excel, the most linear plot [24, 25] of ln k versus 1/T was searched out through regression formula. Values of ln A and E a were obtained from the intercept and slope, respectively, of the plot which corresponded to the model being followed by the step. The plot was based on the following relation:
Here,
The value of ΔS # was approximated using the thermodynamic equation:
where ΔS # is the entropy of activation, A is the frequency factor, R is the gas constant, k is the Boltzmann constant, and T is the peak temperature.
The value of ΔG # was approximated using the thermodynamic relations:
and
On the basis of the findings, 450 °C has been chosen for annealing of the gels.
Annealing, XRD, and magnetic studies
The gels were annealed separately at 450 and 650 °C, respectively, for 1 h in a muffle furnace. Annealing has been done at 650 °C as well in an attempt to obtain nanometric particles of aluminates (MAl2O4) {M = Ni, Zn} and to find out their magnetic properties which could render them useful for chemical sensing applications, etc. By this process, the precursor was thermally decomposed to give the aluminate powder which was later characterized using X-ray diffractometer (XRD Model D/max-IIB, Rigaku) for phase and particle size and Vibrating sample magnetometer(VSM, PAR155) for magnetic parameters observation. The thermal analyses were carried out on Mettler TGA and NETZSCH STA 449F3 instruments. Scherrer formula (D = 0.9 λ/β Cosθ) was used to calculate the particle size [29].
Results and discussion
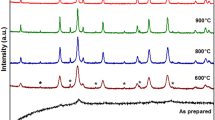
The thermal decomposition of the coprecipitated nickel(II) citrate–aluminum citrate gel in O2 atmosphere is taking place in two steps viz. dehydration and formation of the aluminate. The second step gives an endo peak (−2937 J/g) at 356 °C in the DSC curve. The onset and endset temperatures have been found to be 341.4 and 366.1 °C, respectively. Kinetic/mechanistic analysis of the TG data has also been carried out for the second step. The decomposition is following 3D diffusion mechanism. Values of E a, ΔS #, ΔG #, and ln A were approximated and, found to be 192.278 kJ mol−1, −12.02 J K−1 mol−1, 194.609 kJ mol−1, and 28.76, respectively. The values are in consonance with what is expected during the process. The X-ray diffraction patterns are shown in Figs. 2 and 3 and the magnetization curves are shown in Figs. 4 and 5. The XRD and magnetization data have been summarized in Table 1.
The two samples annealed at 650 °C furnished pure and excellent crystalline phase. The main peaks are indexed in accordance with spinel structure (JCPDS data file# 73-1961) suggesting that the prepared samples are in pure phase with particle size 34 nm in case of ZnAl2O4. The average particle size was found using Scherrer formula [29]. On the basis of kinetic and thermodynamic considerations, formation of aluminate (e.g., MgAl2O4 i.e., MgO + Al2O3) is not expected to take place below 1200 °C [30]. It may be necessary to heat it for several days at 1500 °C. Earlier, nanocrystalline ZnAl2O4 was synthesized (20 nm) by polymer precursor method [31] when the annealing was done at 1000 °C. The coercivity of the sample was reported to be 469 G but the retentivity and magnetization was very small. In the present case, although the ZnAl2O4 obtained after annealing at 450 °C is in amorphous phase, the coercivity was found to be almost the same (470 G). While NiAl2O4 shows poor crystallinity when the annealing temperature is 450 °C, the one obtained at 650 °C shows particle size of 17 nm. The crystallinity of the powders is comparable to the reported value of the aluminates obtained at 1000 °C by the polymer precursor method [31]. Small value of magnetization was found (0.6163 emu/g) for NiAl2O4 annealed at 450 °C.
The magnetic properties are usually determined by the size of the nanocrystallites. The decrease in magnetization with decrease in particle size of nanocrystalline materials is attributed to surface effect, spin canting, and broken exchange bonds [32, 33]. In case of nanocrystalline Ni–Zn ferrite, the magnetization has been found to be increasing from 28.3 to 58.3 emu/g when the annealing temperature is raised from 400 to 1000 °C (2 h) [34]. The degree of magnetization may also be affected by the presence of metallic traces of iron and other magnetic elements [35]. Although aluminum does not have magnetic moment, magnetization has been observed in the present aluminates and the value varies with the annealing temperature.
Conclusions
-
The thermal decomposition of the mixed citrate gel is taking place in two steps viz. dehydration and formation of the aluminate. The second step gives an endo peak (−2937 J/g) at 356 °C in the DSC curve of the coprecipitated nickel(II) citrate–aluminum citrate gel in O2 atmosphere.
-
Although the ZnAl2O4 obtained after annealing at 450 °C is in amorphous phase, the coercivity was found to be almost the same (470 G) as that of literature value of 469 G when the sample was prepared by polymer precursor method followed by annealing at 1000 °C.
-
Annealing at 650 °C leads to particle size of 17 nm (NiAl2O4) and 34 nm (ZnAl2O4), respectively. Thus, the citrate precursor method is good for the synthesis of ZnAl2O4, producing single phase nanocrystalline powder of high quality and crystallinity.
-
Small value of magnetization was found (0.6163 emu/g) for NiAl2O4 annealed at 450 °C.
References
Wilding MC. Aluminates. In: Shackelford JF, Doremus RH, editors. Ceramic and glass materials. New York: Springer; 2008. p. 49–70.
Aitasalo T, Hölsä J, Jungner H, Lastasuaari M, Niittykoski J. Thermoluminescence study of persistent luminescence materials:# Eu2+- and R3+-doped calcium aluminates, CaAl2O4:Eu2+, R3+. J Phys Chem B. 2006;110:4589–98.
Yokota K, Zhang S, Kimura K, Sakamoto A. Eu2+-activated barium magnesium aluminate phosphor for plasma displays–phase relation and mechanism of thermal degradation. J Luminiscence. 2001;92(3):223–7.
Berkstresser GW, Valentino AJ, Brandle CD. Growth of single crystals of lanthanum aluminate. J Cryst Growth. 1991;109:467–71.
Ingram BJ, Mason TO, Asahi R, Park KT, Freeman AJ. Electronic structure and small polaron hole transport of copper aluminate. Phys Rev B. 2001;64:155114–20.
Shimizu K, Maeshima H, Satsuma A, Hattori T. Transition metal-aluminate catalysts for NO reduction by C3H6. Appl Catal B. 1998;18:163–8.
El-Shobaky GA, El-Nabarawy T, Fagal GA. Effect of Li2O doping on solid–solid interactions in the CuO-Al2O3 system. Thermochim Acta. 1989;150:111–20.
Gonsalves LR, Mojumdar SC, Verenkar VMS. Synthesis of cobalt nickel ferrite nanoparticles via autocatalytic decomposition of the precursor. J Therm Anal Calorim. 2010;100:789–92.
Carp O, Patron L, Mindru I, Suciu CJ. Thermal behaviour of some Al(III)–Mg(II) ploynuclear coordination compounds with polycarboxylic acid anions as ligands, precursors of aluminates. J Therm Anal Calorim. 2007;81:77–81.
Valenzuela MA, Bosch P, Zapata B, Aguilar-Ríos G, Lara VH, García-Figueroa E, Schifter I. Effect of hydrogen at high temperature on ZnAl2O4 and Sn-ZnAl2O4. J Therm Anal Calorim. 1995;44:639–53.
El-Shobaky GA, Ghoneim NM, Sultan EA. Thermal decomposition of nickel aluminium mixed hydroxides and formation of nickel aluminate spinel. Thermochim Acta. 1983;63:39–49.
El-Shobaky GA, Ahmed AS, Fagal GA, Mokhtar M. Solid–solid interaction in CuO–ZnO/Al2O3 system under varying conditions. Thermochim Acta. 1998;319:67–74.
MacKenzie KJD, Kemmitt T. Evolution of crystalline aluminates from hybrid-gel derived precursors studied by XRD and multinuclear solid state MAS NMR:I Celsian BaAl2Si2O8. Thermochim Acta. 1999;325:5–12.
Arora BR, Banerjee RK, Rao TSRP, Mandal NK, Bhattacharyya NB, Sen SP. Thermochemical studies on the formation and constitution of zinc oxide-aluminium oxide system. Thermochim Acta. 1973;6:119–28.
Banerjee S, Kumar A, Sujatha Devi P. Preparation of nanoparticles of oxides by the citrate-nitrate process: effect of metal ions on the thermal decomposition characteristics. J Therm Anal Calorim. 2011;104:859–67.
Lazau I, Pacurariu C, Babuta R. The use of thermal analysis in the study of Ca3Al2O6 formation by the polymeric precursor method. J Therm Anal Calorim. 2011;105:427–34.
Milao TM, Oliveira JFA, Araujo VD, Bernardi MIB. Zn0.97M0.03O (M = Co, Fe, and V) pigments: thermal, structural, and optical characterization. J Therm Anal Calorim. 2011;103:873–7.
Lopez-Delgado, Lopez FA, Gonzalo-Delgado L, Lopez-Andres S, Alguacil FJ. Study by DTA/TG of the formation of calcium aluminate obtained from an aluminium hazardous waste. J Therm Anal Calorim. 2010;99:999–1004.
Verma RK, Verma L, Chandra M. Thermoanalytical studies on the non-isothermal dehydration and decomposition of dl-lactates of a series of transition metals. Indian J Chem. 2003;42A:2982–7.
Verma RK, Verma L, Chandra M, Bhushan A. Non-isothermal dehydration and decomposition of dl-lactates of transition metals and alkaline earth metals: A comparative study. J Therm Anal Calorim. 2005;80:351–4.
Brown ME, Gallagher PK. Introduction to recent advances, techniques and applications of thermal analysis and calorimetry. In: Brown ME, Gallagher PK, editors. Hand book of thermal analysis and calorimetry. Amsterdam: Elsevier; 2008. p. 1–12.
Verma RK, Verma L, Chandra M, Verma BP. Kinetic parameters of thermal dehydration and decomposition from thermoanalytical curves of zinc dl-lactate. J Indian Chem Soc. 1998;75:162–4.
Verma RK, Verma L, Ranjan M, Verma BP, Mojumdar SC. Thermal analysis of 2-oxocyclopentanedithiocarboxylato complexes of iron(III), copper(II) and zinc(II) containing pyridine or morpholine as the second ligand. J Therm Anal Calorim. 2008;94:27–31.
Verma RK, Verma L, Bhushan A, Verma BP. Thermal decomposition of complexes of cadmium(II) and mercury(II) with triphenylphosphanes. J Therm Anal Calorim. 2007;90:725–9.
Samtani M, Dollimore D, Alexander KS. Comparison of dolomite decomposition kinetics with related carbonates and the effect of procedural variables on its kinetic parameters. Thermochim Acta. 2002;392–393:135–45.
Bhattacharjee NC, Kumar M, Kumar S, Verma RK. Kinetic and mechanistic studies on non-isothermal decomposition of potassium dioxalatocuprate(II) dihydrate. J Indian Chem Soc. 1998;75(5):317–8.
Kumar M, Verma RK, Verma L, Bhattacharjee NC, Kumar S, Verma BP. Thermal decomposition of potassium trioxalato chromate(III) trihydrate: A kinetic and mechanistic study. Asian J Chem. 1996;8(3):543–6.
Verma BP, Verma RK, Chandra M, Pandey S, Mallick AK, Verma L. A study of non-isothermal decomposition of calcium dl-lactate pentahydrate. Asian J Chem. 1994;6:606–12.
Cullity BD. Elements of X-ray diffraction. London: Addison Wesley; 1978. p. 101.
West AR. Solid state chemistry and its applications. New Delhi: Wiley India; 2007. p. 6–15.
Gama L, Ribeiro MA, Barros BS, Kiminamo RHA, Weber IT, Costa ACFM. Synthesis and characterization of the NiAl2O4, CoAl2O4 and ZnAl2O4 spinels by the polymeric precursors method. J Alloys Compd. 2009;483:453–5.
Coey JMD. Noncollinear spin arrangement in ultrafine ferromagnetic crystallites. Phys Rev Lett. 1971;27:1140–2.
Kodama RH, Berkowitz AE, McNiff EJ. Foners. Surface spin disorder in NiFe2O4 nanoparticles. Phys Rev Lett. 1996;77:394–7.
Caizer C, Stefanescu M. Magnetic characterization of nanocrystalline Ni–Zn ferrite powder prepared by the glyoxalate precursor method. J Phys D. 2002;35:3035–40.
Munoz Mendoza JP, Ayala Valenzuela OE, Corral Flores V, Matutes Aquino J, De la Torre SD. Mechanochemical processing of zinc-ferrite powders and their magnetic characterization. J Alloys Compd. 2005;369:144–7.
Acknowledgements
The authors thank Dr. R. K. Kotnala, Head, Magnetic Lab, National Physical Laboratory (NPL), New Delhi for magnetization measurements and Mr. Mahesh (Mettler) and Ms Priya (Netzsch) for the recording of TG curves. Authors R.K. Singh and A. Yadav also thank Nalanda Open University, Patna for a partial financial support in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, R.K., Yadav, A., Narayan, A. et al. Thermal, XRD, and magnetization studies on ZnAl2O4 and NiAl2O4 spinels, synthesized by citrate precursor method and annealed at 450 and 650 °C. J Therm Anal Calorim 107, 205–210 (2012). https://doi.org/10.1007/s10973-011-1860-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1860-7