Abstract
In this study for the first time the temperature dependences of the heat capacity C 0p and enthalpies of physical transitions of carbosilane dendrimers with diundecylsilyl and diundecylsiloxane terminal groups of the fifth generation have been measured using the methods of precision adiabatic vacuum calorimetry and differential scanning calorimetry over the range from 6 to 580 K. In the above temperature ranges the physical transformations have been detected and their thermodynamic characteristics were estimated and analyzed. The standard thermodynamic functions: heat capacity C 0p (T), enthalpy H°(T) − H°(0), entropy S°(T) − S°(0), and free Gibbs energy G°(T) − H°(0) and standard entropies of formation of dendrimers at T = 298.15 K have been calculated over the range from T → 0 K to 580 K. The thermodynamic properties of studied dendrimers have been compared.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dendritic macromolecules have been intensively studied in the past decade owing to their unique structure and a set of specific characteristics. The synthesis, modification, physicochemical properties, and possible application areas of diverse dendrimers and hyperbranched polymers have been intensively studied [1–5].
At present, particular attention is paid to the characterization of dendrimers since this information is necessary for gaining insight into the structure–properties relationship for these compounds. The dependence of different properties of dendrimers, such as the density, viscosity in solution and bulk, and glass transition temperature, on different parameters of dendritic molecules, primarily, on the nature of terminal groups, has been described in the literature [6–8]. So, to the present time by the methods of precision adiabatic vacuum calorimetry and differential scanning calorimetry standard thermodynamic properties various dendrimers had been investigated in a wide range of temperatures [9–15], in some cases [10–13] their dependences on composition and structure had been revealed and analyzed. Detection of the second high-temperature relaxation transformation for carbosilane dendrimers of high generations became the result of systematic research [13, 14]. The appearance of this transition suggests changes in the character of interactions between dendrimers with an increase in their generation number. It should be noted that the appearance of the second relaxation transition upon passage from the fifth to sixth generation coincides with a change in the aggregation state of dendrimers; that is, dendrimers of the first to the fifth (inclusive) generation are transparent liquids with different viscosities, while from the sixth generation, they have a waxlike consistency. The densification of the surface layer might lead to formation of the physical network between dendrimers. The determination of standard thermodynamic characteristics and its analysis are actual and significant for representatives of new class of macromolecules, i.e., dendrimers.
This research is a part of complex investigations of thermodynamic properties of different homologous series carbosilane dendrimers. It is interesting to evaluate the difference in studying dendrimers structure and composition, notably introduction of siloxane fragment, which insures more mobility of terminal alkyl groups and to begin to systematic investigation of the nature. This gradual variation in the nature of the surface layers enables us to initiate the systematic study of the nature of the second (high-temperature) transition in the temperature dependence of heat capacity.
The aim of this study is to calorimetrically study the temperature dependence of heat capacity of the carbosilane dendrimers with diundecylsilyl and diundecylsiloxane terminal groups of fifth generation in the range 6–(550–580) K, determine of glass transition thermodynamic characteristics and interpret them in terms of physical chemistry, to calculate the standard thermodynamic functions C 0p (T), H°(T) − H°(0), S°(T) − S°(0) and G°(T) − H°(0) over the temperature range from T → 0 to (550–580) K and standard entropies of formation of dendrimers in amorphous (devitrified) state at T = 298.15 K, to compare the thermodynamic properties of studied samples.
Experimental
Samples
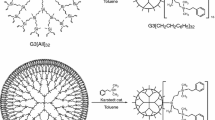
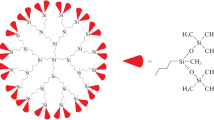
Studied in this work samples of the carbosilane dendrimers with diundecylsilyl and diundecylsiloxane terminal groups of the fifth generation (gross-formulas Si253C3824H8156, M = 61255.8 g mol−1, Si381C4080H8924O128, M = 70747.5 g mol−1, respectively) were synthesized at N.S. Enikolopov Institute of Synthetic Polymer Materials, Russian Academy of Science by the method described in detail elsewhere [16].
The structure of samples and the scheme of their synthesis are presented on Fig. 1.
Under normal conditions the dendrimers are transparent colorless waxy substance. In this study were used samples cleaned by the methods of preparative gel permeation chromatography. The composition and structure of studied samples were confirmed by elemental analysis and methods of NMR 1H-spectroscopy [a “Bruker WP-200 SY spectrometer” (200.13 MHz, a standard is tetramethylsilane)] and IR-spectroscopy (a “Bruker ISF-110” device).
The carbosilane dendrimers with diundecylsilyl and diundecylsiloxane terminal groups of fifth generation were marked as I and II, respectively.
Apparatus and measurement procedure
Heat capacity of studied samples was measured over the range 6–350 K in a BKT-3.0 fully automatic adiabatic vacuum calorimeter with liquid helium and nitrogen used as cooling agents. The ampoule with the substance was filled with dry helium as a heat exchange gas to the pressure of 4 kPa at room temperature. The calorimeter design and measurement procedure are similar to those reported elsewhere [17–19]. The reliability of its operation was tested by measuring the heat capacity of special purity copper, standard synthetic corundum, and K-3 benzoic acid prepared at the D.I. Mendeleev All-Russian Institute for Metrology (VNIIM). It was established by the calibration that the determination of the heat capacity C 0p of substances was measured with an error not exceeding ±2% at T = (6–15) K, ±0.5% between 15 and 40 K, and ±0.2% in the range from 40 to 350 K. The phase transition temperatures are measured within about ±0.01 K and the enthalpies of transformations with the error of ±0.2%.
An automatic thermoanalytical system operating on the principle of triple thermal bridge, differential scanning calorimeter (ADCTTB) [20, 21] was used to study the temperature dependence of the heat capacity C 0p = f(T) over the temperature range 330–580 K. The reliability of the calorimeter operation was checked by measuring the heat capacity of the standard sample of synthetic corundum, special purity copper as well as the thermodynamic characteristics of fusion of indium, tin, and lead. As a result, it was found that the calorimeter and the measurement technique allow one to obtain the heat capacity C 0p values of the substances with an error of ±2% and the phase transformation temperatures and enthalpies within to ca. ±0.5 K and ±1%. Since the heat capacity of the examined substance was also measured between 330 and 350 K in the adiabatic vacuum calorimeter and the conditions of measurements in the ADCTTB were special chosen. It should be noted that scanning was obtained by slow speed of heating and cooling and the infill of the calorimetric ampoule was close to 100%. These manipulations are allowed determine the results coincidence from different methods with the permissible error.
Results and discussion
Heat capacity
Experimental values of heat capacity over the range from 6.37 to 549.6 K and 6.50 to 580.4 K and the smoothed C 0p = f(T) plots for I and II, respectively (Tables 1, 2), are illustrated in Figs. 2 and 3. The masses of I and II samples located in the calorimetric ampoule of the adiabatic calorimeter were 0.2391 and 0.2250 g and those placed in the dynamic one were 0.1395 and 0.1586 g, respectively. 279 experimental C 0p values were obtained for I and 286 for II in three series of experiments in BCT. The heat capacity of the dendrimers was everywhere between 15 and 50% of the overall heat capacity of the calorimetric ampoule with the substance. The experimental C 0p values were smoothed by means of a computer program in the form of degree and semi-logarithmic polynomials. As an example, the polynomials with the corresponding coefficients for ranges from 6 to 13 K and from 225 to 346 K are cited below. For II in the interval between 6 and 13 K, the equation C 0p (T) = −1.10542 × 102 + 2.14308 × 103(T/30) − 1.74246 × 104(T/30)2 + 7.77548 × 104(T/30)3 − 2.06761 × 105(T/30)4 + 3.31179 × 105(T/30)5 − 2.98584 × 105(T/30)6 + 1.17637 × 105(T/30)7 as well as the equation C 0p (T) = −1.7409054 × 105 + 1.3729883 × 105(T/30) − 4.6048614 × 104(T/30)2 + 8.5233573 × 103(T/30)3 − 9.4061029 × 102(T/30)4 + 6.1906776 × 10(T/30)5 − 2.2505345(T/30)6 + 3.4870097 × 10−2(T/30)7 in the range from 225 to 346 K were used. In these equations, C 0p is in kJ mol−1 K−1 and temperature is in K. The root-mean-square deviation of the C 0p points from the corresponding smoothed curve C 0p = f(T) was ±0.20% over the range 6–13 K, ±0.50% from 10 to 40 K, ±0.20% between 30 and 100 K, in the ranges 90–186 and 225–346 K the root-mean-square deviation was ±0.40 and ±0.40% in the interval from 330 to 486 K.
The heat capacity of I smoothly and quite regularly changes with temperature increase in the range 6–195 K. There is anomalous changing the heat capacity with temperature rise on the temperature dependence C 0p = f(T) in the ranges 195–235 and 461–532 K (Fig. 2). Since 195 K the heat capacity is sharply increased with growth of temperature, achieving the maximal meaning C 0p,max = 330.8 kJ mol K−1 at 226.48 K (Fig. 2, the point D), then sharply decreases up to 119.1 kJ mol K−1 at 235.07 K. The above transformation is presumably related to devitrification of the amorphous part of the polymer, which smoothly passes to melting of its crystalline part.
It should be noted that the temperature of devitrification for studied sample is much higher in comparison with investigated earlier dendrimers of the sixth [13], seventh, and ninth [14] generations with terminal butyl groups and dendrimers by first–fifth generations with terminal allyl groups [11]. Probably, it is caused by a various nature of terminal groups, and also ability of undecyl fragments to form the ordered crystal areas, as well as in the case of methoxyphenyl benzoate-terminated liquid crystalline dendrimers [15].
In the range 461–532 K there is the second transformation on a curve C 0p = f(T) reminding on the form the glass transition. The similar jump of the heat capacity was observed for studied earlier carbosilane dendrimers with terminal butyl groups: in range 370–470 K for the sixth generation (G-6 (Bu)256) [13] and in an interval 390–490 K for seventh (G-7 (Bu)512) and ninth (G-9 (Bu)2048) generations [14]. It is visible that the observable transition as well as the glass transition come at higher temperatures than for compared dendrimers. The assignment of the transition undoubtedly requires more extensive thermophysical, thermomechanical, and rheological experiments. Its physicochemical interpretation and assignment to a specific group according to thermodynamic classification likewise needs additional studies. However, the shift of the second transition toward higher temperatures with an increase in the size of terminal groups from butyl to undecyl is consistent with our hypothesis that there is a physical network that is similar to the entanglement network arising in classical polymers.
The temperature dependence of the heat capacity C 0p = f(T) for II (Fig. 3) is similar considered for I; however, the glass transition and melting temperature (Table 3) are lower for the dendrimer containing the silicone spacer than those for the poly(undecyl) dendrimer. This situation is most likely related to the presence of the flexible siloxane spacer between the alkyl decoration and the dendrimer core. The jump of the heat capacity, corresponding to the second transformation, is somewhat lower, than in the case of I.
Low-temperature heat capacity (20 ≤ (T/K) ≤ 50) of the dendrimers under study is linear function versus temperature. This indicates on chain (linear) topology structure of compounds [22, 23].
The glass transition and glassy state
The thermodynamic characteristics of devitrification and glassy state of the dendrimers are listed in Table 3. The glass transition temperature T 0g was determined by the Alford and Dole method [24–26] from the inflection of the plot of the temperature dependence of entropy of heating. The devitrification intervals and an increase in the heat capacity on devitrification were determined graphically. The configuration entropy was calculated by Eq. 1:
where T 02 is Kauzmann temperature [27], the ratio T 0g /T 02 is equal to (1.29 ± 0.14) [28] and [29]. It is suggested that the ratio is valid also for all dendrimers under study. It was shown [28] and [30] that the value S 0conf is close to the S 0(0) value. Taking this into account it was assumed quite normally that S 0(0) = S 0conf [29] to evaluate the absolute value of the absolute entropy.
Melting
In Table 4 are represented the thermodynamic quantities of melting. The melting temperature of a crystalline part of samples accepted equal to temperature of the end of transformation (Figs. 2, 3, point D). The enthalpies of melting were determined as a difference of integrals on temperature under curves apparent (BCDEB) and normal (BB’CEB, extrapolational) heat capacity, respectively, in the interval of transformation.
Standard thermodynamic functions
To calculate the standard thermodynamic functions (Tables 5, 6) of I and II, their C 0p values were extrapolated from 6 K to zero temperature consistently with the Debye law in the low-temperature limit [30]:
where D denotes Debye function of the heat capacity, n and θ D are specially selected parameters (with n = 3, θD = 51.7 and 63.9 K for I and II, respectively). Equation 2 with these parameters describes the experimental C 0p values of the compound between 6 and 12 K with the error of ±1.5%. In calculating the functions, it was assumed that Eq. 2 reproduced C 0p values of I and II at T < 6 K with the same error.
The calculation of enthalpy and entropy was made by the numerical integration of C 0p = f(T) and C 0p = ln f(T) curves, respectively. The free Gibbs energy was calculated with Gibbs–Helmholtz equation from the enthalpies and entropies at the corresponding temperatures [31]. It was suggested that the error of the function values was ±2% at T < 15 K, ±0.5% between 15 and 40 K, ±0.2% in the range from 40 to 350 K, and ±2% from 330 to 580 K.
Standard entropies of formation of dendrimers at 298.15 K
From the absolute values of entropies (Tables 5, 6) of studied dendrimers at 298.15 K and the absolute entropies of simple substances [C(gr), Si(cr)] [32] and [H2(g), O2(g)] [33] at T = 298.15 K it was found that the standard entropies of formation of dendrimers are −418.9, −466.3 kJ/(K mol), respectively. The values conform to the equations:
where the physical states of the reagents are indicated in parentheses (gr stands for graphite, g for gas, cr for crystal, and a for amorphous). The large negative Δf S 0 values are related apparently to binding of 4078 and 4462 mol of gaseous hydrogen in the reaction of formation of dendrimers from simple substances.
Comparison of thermodynamic properties of studied dendrimers
It was interesting to compare thermodynamic characteristics of studied dendrimers. The values of glass transition and fusion temperature (Tables 3, 4) for dendrimer with siloxane outer layer are lower than for poly(undecyl) dendrimer. Most probably it is connected with presence in structure of this dendrimer flexible silicone spacer between alkyl decoration and dendrimer core. This small difference in structure of studied dendrimers imparts higher mobility to terminal alkyl groups owing to the presence of a more flexible linkage between the surface structure and the dendrimer core. The jump of the heat capacity corresponding to second transition is somewhat lower than in the case of dendrimer I, while molecular mass is noticeably higher owing to incorporation of siloxane spacers. Take into account that dendrimers I and II have similar cores and nature of outer layers it is possible to state that the strength of entanglements depends on the flexibility of outer structural fragments. In other words, the incorporation of flexible siloxane spacers does not change the chemical nature of outer layers but changes their flexibility and thus makes the entanglements less efficient.
Conclusions
-
The heat capacities of carbosilane dendrimers with diundecylsilyl and diundecylsiloxane terminal groups of the fifth generation have been measured over the range from 6 to (550–580) K.
-
From experimental data the standard thermodynamic functions of studied carbosilane dendrimers, namely, the heat capacity C 0p (T), enthalpy H°(T) − H°(0), entropy S°(T) − S°(0), and Gibbs function G°(T) − H°(0) have been calculated over the range from T → 0 to (550–580) K, and the values of standard thermodynamic functions of formation of studied dendrimers at T = 298.15 K have been calculated.
-
The standard thermodynamic properties of studied dendrimers have been compared; as a result some dependences thermodynamic properties on composition and structure were obtained.
References
Tomalia DA, Naylor AM, Goddart WA. Starburst dendrimers: molecular-level control of size, shape, surface chemistry, topology, and flexibility from atoms to macroscopic matter. Angew Chem Int Ed Engl. 1990;29:138–75.
Newkome GR. Advances in dendritic macromolecules. Greenwich: JAI Press; 1994.
Astruc D, Chardac F. Dendritic catalysts and dendrimers in catalysis. Chem Rev. 2001;101:2991–3023.
Muzafarov AM, Rebrov EA, Papkov VS. Three-dimensionally growing polyorganosiloxanes. Possibilities of molecular construction in highly functional systems. Russ Chem Rev. 1991;60:807–14.
Beletskaya IP, Chuchuryukin AV. Synthesis and properties of functionalized dendrimers. Russ Chem Rev. 2000;69:639–60.
Lorenz K, Mulhaupt R, Frey H, Rapp U. Mayer-Posner F.J. Carbosilane-based dendritic polyols. Macromolecules. 1995;28:6657–61.
Wooley KL, Hawker CJ, Pochan JM, Frechet GMJ. Physical properties of dendritic macromolecules: a study of glass transition temperature. Macromolecules. 1993;26:1514–9.
Lorenz K, Frey H, Stuhn B, Mulhaupt R. Carbosilane dendrimers with perfluoroalkyl end groups. Core–shell macromolecules with generation-dependent. order macromolecules. 1997;30:6860–8.
Lebedev BV, Smirnova NN, Ryabkov MV, Ponomarenko SA, Makeev EA, Boiko NI, Shibaev VP. Thermodynamic properties of carbosilane dendrimer of the first generation with terminal methoxyundecylenate groups in the range 0–340 K. Polym Sci Ser A. 2001;43:323–31.
Ryabkov MV, Kulagina TG, Lebedev BV. Thermodynamic properties of carbosilane dendrimers of the first and second generations with terminal allyl groups in the range 0–340 K. Russ J Phys Chem. 2001;75:1988–96.
Lebedev BV, Ryabkov MV, Tatarinova EA, Rebrov EA, Muzafarov AM. Thermodynamic properties of the first to fifth generations of carbosilane dendrimers with allyl terminal groups. Russ Chem Bull. 2003;52:545–51.
Smirnova NN, Lebedev BV, Khramova NM, Tsvetkova LYa, Tatarinova EA, Myakushev VD, Muzafarov AM. The thermodynamic properties of carbosilane dendrimers of the sixth and seventh generations with terminal groups in the temperature range 6–340 K. Russ J Phys Chem. 2004;78:1369–74.
Smirnova NN, Stepanova OV, Bykova TA, Muzafarov AM, Tatarinova EA, Myakushev VD. Thermodynamic properties of carbosilane dendrimers of the third to the sixth generations with terminal butyl groups in the range from T → 0 to 600 K. Thermochim Acta. 2006;440:188–94.
Smirnova NN, Stepanova OV, Bykova TA, Markin AV, Tatarinova EA, Muzafarov AM. Thermodynamic properties of carbosilane dendrimers of the seventh and ninth generations with terminal butyl groups in the temperature range from T → 0 to 600 K. Russ Chem Bull. 2007;56:1991–5.
Lebedev BV, Kulagina TG, Ryabkov MV, Ponomarenko SA, Makeev EA, Boiko NI, Shibaev VP, Rebrov EA, Musafarov AM. Carbosilane dendrimer of second generation with terminal methoxyundecylenate groups. J Therm Anal Calorim. 2003;71:481–92.
Tereshchenko AS, Tupitsyna GS, Tatarinova EA, Bystrova AV, Muzafarov AM, Smirnova NN, Markin AV. Carbosilane dendrimers with diundecylsilyl, diundecylsiloxane, and tetrasiloxane terminal groups: synthesis and properties. Polym Sci Ser B. 2010;52:41–8.
Varushchenko RM, Druzhinina AI, Sorkin EL. Low-temperature heat capacity of 1-bromoperfluorooctane. J Chem Thermodyn. 1997;29:623–7.
Malyshev VM, Milner GA, Sorkin EL, Shibakin VF. Automatic low-temperature calorimeter. Pribory i Tekhnika Eksperimenta. 1985;6:195–7.
Paukov IE, Kovalevskaya YA, Kiseleva IA, Shuriga TN. A low-temperature heat capacity study of natural lithium micas. J Therm Anal Calorim. 2010;992:709–12.
Yagfarov MSh. Novii metod izmerenia teploemkostei i teplovih effektov. Zh Fiz Khimii. 1969;43:1620–5.
Kabo AG, Diky VV. Details of calibration of a scanning calorimeter of the triple heat bridge type. Thermochim Acta. 2000;347:79–84.
Lazarev VB, Izotov AD, Gavrichev KS, Shebershneva OV. Fractal model of heat capacity for substances with diamond-like structures. Thermochim Acta. 1995;269–270:109–16.
Tarasov VV. Theory of heat capacity of chain and layer structures. Zhurnal fizicheskoi khimii. 1950;24:111–28.
Alford S, Dole M. Specific heat of synthetic high polymers. VI. Study of the glass transition in polyvinyl chloride. J Chem Soc. 1955;77:4774–7.
Smirnova NN, Lebedev BV, Bykova TA, Markin AV, Tur DR. Thermodynamic properties of poly-[bis(trifluoroetoxy)-phosphazene] in the range from T → 0 to 620 K. J Therm Anal Calorim. 2009;95:229–34.
Wunderlich B. Thermodynamic description of condensed phases. J Therm Anal Calorim. 2010;102:413–24.
Kauzmann W. The Nature of the glassy state and the behavior of liquids at low temperatures. Chem Rev. 1948;43:219–56.
Adam G, Gibbs JU. On the temperature dependence of cooperative relaxation properties in glass-forming liquids. J Chem Phys. 1965;43:139–46.
Bestul A, Chang SS. Excess entropy at glass transformation. J Chem Phys. 1964;40:3731–3.
Rabinovich IB, Nistratov VP, Telnoy VI, Sheiman MS. Thermochemical and thermodynamic properties of organometallic compounds. New York: Begell House Inc. Publishers; 1999.
Lebedev BV. Thermodynamics of polymers. Gorky: Gorky State University; 1989.
Cox JD, Wagman DD, Medvedev VA. Codata key values for thermodynamics. New York; 1984. Database http://webbook.nist.gov/chemistry/.
Chase MW Jr. NIST-JANAF themochemical tables. 4th ed. J Phys Chem Ref Data Monogr. 1998;9:1951. Database http://webbook.nist.gov/chemistry/.
Acknowledgements
This study was financially supported by the Russian Foundation for Basic Research (project 08-03-00214a).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Markin, A.V., Samosudova, Y.S., Smirnova, N.N. et al. Thermodynamics of carbosilane dendrimers with diundecylsilyl and diundecylsiloxane terminal groups. J Therm Anal Calorim 105, 663–676 (2011). https://doi.org/10.1007/s10973-010-1199-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-1199-5