Abstract
The heat capacity–temperature dependence for a fourth-generation carbosilane dendrimer with terminal trimethylsilylsiloxane groups is determined via adiabatic vacuum calorimetry in the 5–344 K range of temperatures and differential scanning calorimetry in the 310–560 K range of temperatures. An anomalous change in the heat capacity of the dendrimers in the range of T = 46–68 K is detected along with a transition in the range of T = 179–196 K, due to its devitrification. The thermodynamic characteristics of the detected transformations are determined and analyzed. The standard thermodynamic functions of the studied dendrimers in the range T → 0 to 560 K and the standard entropy of its formation at T = 298.15 K are calculated using the obtained experimental data. A comparative analysis is performed of the thermodynamic properties of fourth-generation dendrimers that differ in the nature of their molecular skeletons and terminal groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Dendrimers are nanosized spherical macromolecules characterized by monodisperse distributions (compared to classical polymers) and hyperbranched three-dimensional architectures. The main structural elements of dendrimers include a core (the initial multifunctional molecule), an inner sphere (exponentially repeating units resulting in the formation of G1, G2, G3, and higher generations), and an outer layer (terminal functional groups located on the surfaces of macromolecules and growing exponentially according to the number of the generation) [1–3].
Dendrimers are objects of intense fundamental and applied research, due to their highly ordered controlled structure and set of unique properties [4–8]. The precise geometry of macromolecules and the variability in the number of catalytic sites, which opens up new ways of controlling the mechanisms of chemical reactions, make dendrimers promising for use as catalysts [9, 10]. Dendrimers also have good solubility and biological inertness, so they can act as molecular containers for the targeted delivery of anticancer drugs. Results from biomedical studies of dendrimers are close to being put to use [11–14]. The development of materials for photonic and molecular electronics is based on dendrimers being an ensemble of macromolecules that is capable of self-assembly and resistant to chemicals, mechanical action, and photo oxidation [15–17].
Identifying a set of standard thermodynamic characteristics for dendrimers with different natures of their cores and surface layers via precision calorimetry in a wide range of temperatures allows us to establish and analyze relationships between compositions and structural properties that are of practical importance for these compounds [18–29]. Data on the thermodynamic properties of dendrimers serve as a theoretical basis in developing processes for preparing promising dendrimer-based nanomaterials.
The aim of this work was to perform a calorimetric study of a fourth-generation carbosilane dendrimer with terminal trimethylsilylsiloxane groups in the 5–560 K range of temperatures. It is a continuation of earlier studies to find the heat capacity of the dendrimer in the above range of temperatures; detect possible physical transformations and determine their thermodynamic characteristics; calculate the standard thermodynamic functions of the dendrimer in the range of T → 0 to T = 560 K and the standard entropy of its formation from elementary substances at T = 298.15 K; and perform a comparative analysis of the thermodynamic properties of fourth-generation dendrimers that differ in the nature of their molecular skeletons and terminal groups.
EXPERIMENTAL
Sample Characteristics
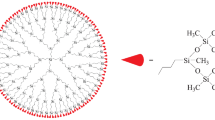
Figure 1 shows the structure of the studied fourth-generation carbosilane dendrimers with terminal trimethylsilylsiloxane groups G4[OSi(CH3)3]64, where G4 is the number of the generation of the dendrimer and [OSi(CH3)3]64 denotes the fragment of the terminal groups of the dendrimer and their number. The sample was synthesized at the Enikolopov Institute of Synthetic Polymer Materials (Moscow, Russia). The polymer matrix for preparing the target dendrimer was a third-generation carbosilane dendrimer with terminal diallylmethylsilyl groups. Its outer layer was modified via hydrosilylation with Karstedt catalyst at room temperature. The modifier was 1,1,1,3,5,5,5-heptamethyltrisiloxane [30]. The composition and structure of the dendrimer were confirmed via elemental analysis, 1H, 13C, 29Si NMR spectroscopy, and small-angle X-ray scattering. The intramolecular structure and macroscopic characteristics of the G4[OSi(CH3)3]64 dendrimer were estimated by means of molecular dynamics using atomistic models. According to data from preparative-scale chromatography, the level of the main substance in the dendrimer was around 99 mol %. The molar weight of the G4[OSi(CH3)3]64 carbosilane dendrimer (M(C432H1116O64Si125) = 10 848.1 g/mol) was calculated using the Table of Standard Atomic Weights as recommended by IUPAC [31].
Apparatus and Measuring Procedure
Our thermogravimetric (TG) analysis of the G4[OSi(CH3)3]64 carbosilane dendrimer was performed on a TG 209 F1 Iris thermobalance (NETZSCH, Germany) in the 300–800 K range of temperatures (purge gas, high-purity argon; gas flow rate, 25 mL min−1). The weight of the dendrimer charge in an aluminum crucible was 17.653 mg; the crucible containing the substance was heated at a rate of 5 K min−1. TG data showed that the initial decomposition temperature of the studied dendrimer was T = 560 K (weight loss, 2%). The resulting TG curve for the G4[OSi(CH3)3]64 dendrimer is shown in Fig. 2.
The heat capacity–temperature dependence for the G4[OSi(CH3)3]64 carbosilane dendrimer in the 5–344 K range of temperatures was determined using a BKT-3 adiabatic vacuum calorimeter (TERMIS, Russia). The design of the calorimeter and the measuring procedure were described in [32, 33]. Each sample of the G4[OSi(CH3)3]64 dendrimer (m = 0.1579 g) was weighed on a Shimadzu AUX 220 analytical balance (Japan) and placed in a titanium calorimetric ampule with thin walls. Prior to heat capacity measurements, small amounts of special purity dry helium gas (p ~ 5 kPa) were added to improve the heat conductivity of the calorimetric system. The cooling agents were liquid helium and nitrogen in the 5–85 and 83–344 K ranges of temperature, respectively. The rate of heating of the ampule containing the substance was 0.2 K min−1. Calorimetric measurements were made twice in the temperature ranges where physical transformations of the dendrimer were observed. The reliability of the instrument was verified by measuring the heat capacities of reference samples of benzoic acid, synthetic sapphire, and high-purity copper in the 5–350 K range of temperatures [34]. The adiabatic calorimeter was found to allow determination of the heat capacities of compounds with relative expanded uncertainty Ur(Cp) = 0.02 in the 5–15 K range of temperatures, Ur(Cp) = 0.005 in the 15–40 K range of temperatures, and Ur(Cp) = 0.002 in the 40–350 K range of temperatures. The temperatures and enthalpies of phase and physical transformations were determined with standard uncertainty u(Ttr) = 0.02 K and cumulative relative expanded uncertainty Uc,r(ΔtrH) = 0.01, respectively.
The heat capacity of the G4[OSi(CH3)3]64 carbosilane dendrimer in the 310–560 K range of temperatures was determined using a DSC 204 F1 Phoenix differential scanning calorimeter (NETZSCH, Germany). The experimental procedure and the design of the instrument were described in [35, 36]. The temperature and heat flow of the calorimeter were calibrated by determining the temperatures and enthalpies of melting of high-purity (99.99%) reference samples of indium, bismuth, tin, mercury, biphenyl, and cyclohexane [37]. The calibration experiments were performed at a heating rate of 5 K min−1; the purge gas was high-purity argon with a flow rate of 25 mL min–1. It was found that DSC allows the temperatures and enthalpies of phase and physical transformations to be determined with standard uncertainty u(Ttr) = 0.5 K and cumulative relative expanded uncertainty Uc,r(ΔtrH) = 0.01, respectively.
Determining the heat capacity via DSC required three successive measurements of [38]
• the baseline (a reference empty crucible + an empty crucible for each sample);
• a standard sample of α-Al2O3 sapphire (a reference empty crucible + a crucible with a sample of the sapphire);
• each considered sample (a reference empty crucible + a crucible with a sample of the dendrimer).
All DSC measurements were made in the 310–560 K range of temperatures at a heating rate of 5 K min−1 (purge gas, high-purity argon; gas flow rate, 25 mL min−1). The weight of each G4[OSi(CH3)3]64 dendrimer sample placed in an aluminum crucible for DSC measurements was 16.82 mg. The heat capacity of the dendrimer was determined according to ratios, according to the procedure described in the international ISO 11357-4:2021, ASTM E1269-11(2018), and DIN 51007:2019-04 standards. The resulting data were analyzed and processed using the NETZSCH Proteus Software program. DSC was found to allow determination of the heat capacities of substances with relative expanded uncertainty Ur(Cp) = 0.02 in the 310–560 K range of temperatures.
RESULTS AND DISCUSSION
Heat Capacity
The heat capacity–temperature curve for the G4[OSi(CH3)3]64 carbosilane dendrimer is shown in Fig. 3. Experimental values of the dendrimer’s heat capacity Cp,m are given in Table 1 (Series 1–4 were obtained using an adiabatic vacuum calorimeter; Series 5 was obtained using DSC).
Standard Thermodynamic Characteristics of the Low-Temperature Heat Capacity Anomaly
The studied dendrimer was cooled from room temperature to the initial measuring temperature (T = 5 K) at a rate of 0.02 K s–1. An anomalous change in the heat capacity of the sample was detected upon heating it in the range of T = (46–68) K (Fig. 4). The change was expressed as a positive deviation from the normal (interpolating) run of the curve. Similar anomalies were detected earlier in younger generations of carbosilane dendrimers with different terminal groups in the same range of temperatures. Such anomalies are systemic in nature. They are governed by the number of the dendrimer’s generation, and are virtually independent of the nature of molecular skeleton and terminal groups. Combined calorimetric and spectral studies of several younger generations of dendrimers [18–21, 24–29] suggest that such transformations are due to thin structural (conformational) vibrations of methyl groups in dendrimer macromolecules when they are heated. As has been noted in the literature, such low-temperature anomalies should be attributed to equilibrium relaxation transitions of the order ⇄ disorder type, according to the Westrum–McCallaf thermodynamic classification.
Calculated thermodynamic characteristics of low-temperature anomalies of fourth-generation dendrimers are given in Table 2. The range of ΔT was determined from the heat capacity–temperature dependence. The points at which the anomalous heat capacity dependence starts and ends were taken as the initial (Tinit) and final (Tfin) temperatures of transition. Enthalpy ΔtrH° was calculated as the difference between integrals over curves \(C_{\text{p}}^{^\circ }\) = f(T) of apparent and normal heat capacities of the substance in the range of anomalies. Entropy ΔtrS° was calculated in a similar manner using the \(C_{\text{p}}^{^\circ }\) = f(\(\ln T\)) curve.
Standard Thermodynamic Characteristics of Devitrification and the Glassy State
The dendrimer is devitrified when heated in the range of T = (179–196) K (Fig. 3, BF section). The detected transition was reproduced upon cooling and reheating in the same range of temperatures.
The thermodynamic characteristics of devitrification and the glassy state of the dendrimer include temperature \(T_{\text{g}}^{^\circ }\) of devitrification, range ΔT of the temperature of devitrification, change (increase) \(\Delta C_{\text{p}}^{^\circ }(T_{\text{g}}^{^\circ })\) in heat capacity upon devitrification, configurational entropy \(S_{\text{conf}}^{^\circ }\), and residual entropy S°(0). Data obtained for the studied dendrimer and the available data for fourth-generation dendrimers are given in Table 3. Temperature \(T_{\text{g}}^{^\circ }\) of devitrification was defined as the point where three tangents to the \(C_{\text{p}}^{^\circ }\) = f(T) curve in the range of devitrification intersected. Range ΔT of devitrification and change \(\Delta C_{\text{p}}^{^\circ }(T_{\text{g}}^{^\circ })\) in heat capacity upon devitrification were determined graphically. Configurational entropy \(S_{\text{conf}}^{^\circ }\) was calculated according to the equation proposed in [39]:
where TK is the Kauzmann temperature [40] and the \((T_{\text{g}}^{^\circ }{\text{/}}{{T}_{\text{K}}})\) ratio is 1.29 [41]. \(S_{\text{conf}}^{^\circ }\) was calculated by assuming the above ratio to be true for the studied compound. In determining the absolute entropy of the dendrimer, we assumed that \(S_{\text{conf}}^{^\circ }\) = S°(0).
We may conclude from our comparative analysis of data obtained in this work and earlier that the temperature of dendrimer devitrification depends on both the chemical structure of the outer layer groups and the structure of the core. The temperature of devitrification was 176 K for the G4[OSi(CH3)3]48 siloxane dendrimer with three branches from the central silicon atom (Fig. 5a). This low \(T_{\text{g}}^{^\circ }\) value was due to flexible siloxane fragments being in the inner sphere and on the surface layer of the dendrimer [29]. The studied fourth-generation carbosilane dendrimers had four branches from the central silicon atom, reducing their molecular mobility and raising their temperatures of devitrification relative to the siloxane dendrimer. Such a tendency was also observed for the G4[OSi(CH3)3]64 dendrimer obtained in the this work (\(T_{\text{g}}^{^\circ }\) = 191 K), and for carbosilane dendrimer G4[But]64 with terminal butyl groups (\(T_{\text{g}}^{^\circ }\) = 186 K) [19]. The liquid-crystalline fourth-generation dendrimer with terminal methoxyphenyl benzoate mesogenic groups G4[Und-MPhB]64 had the highest temperature of devitrification (\(T_{\text{g}}^{^\circ }\) = 258 K) (Fig. 5b), which is explained by strong orientation interactions between them and thus the high rigidity of the dendrimer molecule in general [23]. The change in the chemical nature of the dendrimer molecular skeleton and surface layer is therefore an effective tool for controlling their different physicochemical characteristics.
Standard Thermodynamic Functions
The \(C_{\text{p}}^{^\circ }\) = f(T) curve was fitted using logarithmic polynomials and then extrapolated from the initial measurement temperature to T → 0 using the Debye heat capacity function [42]
where D is the Debye function and n = 83 and ΘD = 34.97 K are tailor-made parameters. Equation (2) with the above parameters describes the experimental \(C_{\text{p}}^{^\circ }\) values of the dendrimer in the range of T = (6–9) K with an error of ±1.3%.
The standard thermodynamic functions of the studied G4[OSi(CH3)3]64 carbosilane dendrimer were calculated from the obtained values (Table 4) by assuming that Eq. 2 reproduces values with an error of ±1.3% when T ≤ 6 K. Enthalpy [H°(T) − H°(0)] and entropy [S°(T) − S°(0)] were calculated by numerically integrating functions \(C_{\text{p}}^{^\circ }\) = f(T) and \(C_{\text{p}}^{^\circ }\) = f(ln T), respectively. Gibbs energy [G°(T) − H°(0)] was calculated using the Gibbs–Helmholtz equation
A detailed procedure for calculating standard thermodynamic functions was published in [43].
Using the values of [S°(T) – S°(0)] at T = 298.15 K (Table 4) and the residual entropy for the studied dendrimer (Table 3) and the absolute entropies of elementary substances (C(gr), H2(g), O2(g), Si(c) [44]), we calculated the standard entropy of formation ΔfS° of the carbosilane dendrimer in the amorphous (devitrified) state at the same temperature. Resulting value ΔfS°(С432Н1116O64Si125, 298.15) = −60 699 ± 298 J K−1 mol−1 corresponds to the equation
where (gr) is graphite, (g) is gas, (c) is crystal, and (dev) is the devitrified state.
REFERENCES
Dendrimers and Other Dendritic Polymers, Ed. by J. M. J. Fréchet and D. A. Tomalia (Wiley, Chichester, UK, 2001).
G. R. Newkome, C. N. Moorefield, and F. Vögtle, Dendrimers and Dendrons: Concepts, Syntheses, Applications (Wiley-VCH, Weinheim, 2002).
F. Vögtle, G. Richardt, and N. Werner, Dendrimer Chemistry: Concepts, Syntheses, Properties, Applications (Wiley-VCH, Weinheim, 2009).
A. W. Bosman, H. M. Janssen, and E. W. Meijer, Chem. Rev. 99, 1665 (1999).
D. A. Tomalia, Prog. Polym. Sci. 30, 294 (2005).
A. M. Muzafarov and E. A. Rebrov, J. Polym. Sci. A 46, 4935 (2008).
L. M. Bronstein and Z. B. Shifrina, Chem. Rev. 111, 5301 (2011).
A. M. Muzafarov, N. G. Vasilenko, E. A. Tatarinova, G. M. Ignat’eva, V. M. Myakushev, M. A. Obrezkova, I. B. Meshkov, N. V. Voronina, and O. V. Novozhilov, Polymer Sci., Ser. C 53, 48 (2011).
R. van Heerbeek, P. C. J. Kamer, P. W. N. M. van Leeuwen, et al., Chem. Rev. 102, 3717 (2002).
D. Astruc, E. Boisselier, and C. Ornelas, Chem. Rev. 110, 1857 (2010).
S. Svenson and D. A. Tomalia, Adv. Drug Deliv. Rev. 57, 2106 (2005).
M. A. Mintzer and M. W. Grinstaff, Chem. Soc. Rev. 40, 173 (2011).
J. Yang, Q. Zhang, H. Chang, et al., Chem. Rev. 115, 5274 (2015).
E. Pędziwiatr-Werbicka, K. Milowska, V. Dzmitruk, et al., Eur. Polym. J. 119, 61 (2019).
M. J. Cho, D. H. Choi, P. A. Sullivan, et al., Prog. Polym. Sci. 33, 1013 (2008).
W. Wu, C. Ye, J. Qin, et al., ACS Appl. Mater. Interfaces 5, 7033 (2013).
X. Zhang, Y. Zeng, T. Yu, et al., J. Phys. Chem. Lett. 5, 2340 (2014).
B. V. Lebedev, M. V. Ryabkov, E. A. Tatarinova, et al., Russ. Chem. Bull. 52, 545 (2003).
N. N. Smirnova, O. V. Stepanova, T. A. Bykova, et al., Thermochim. Acta 440, 188 (2006).
N. N. Smirnova, A. V. Markin, Ya. S. Samosudova, G. M. Ignat’eva, E. Yu. Katarzhnova, and A. M. Muzafarov, Russ. J. Phys. Chem. A 87, 552 (2013).
A. V. Markin, S. S. Sologubov, N. N. Smirnova, et al., Thermochim. Acta 617, 144 (2015).
S. S. Sologubov, A. V. Markin, N. N. Smirnova, et al., J. Phys. Chem. B 119, 14527 (2015).
Ya. S. Samosudova, A. V. Markin, N. N. Smirnova, et al., J. Chem. Thermodyn. 98, 33 (2016).
S. S. Sologubov, A. V. Markin, N. N. Smirnova, et al., J. Therm. Anal. Calorim. 125, 595 (2016).
N. N. Smirnova, S. S. Sologubov, Yu. A. Sarmini, A. V. Markin, N. A. Novozhilova, E. A. Tatarinova, and A. M. Muzafarov, Russ. J. Phys. Chem. A 91, 2317 (2017).
S. S. Sologubov, A. V. Markin, N. N. Smirnova, N. A. Novozhilova, E. A. Tatarinova, and A. M. Muzafarov, Russ. J. Phys. Chem. A 92, 235 (2018).
S. S. Sologubov, A. V. Markin, Yu. A. Sarmini, et al., J. Therm. Anal. Calorim. 138, 3301 (2019).
A. V. Markin, Yu. A. Sarmini, S. S. Sologubov, N. N. Smirnova, K. L. Boldyrev, E. A. Tatarinova, I. B. Meshkov, and A. M. Muzafarov, Russ. J. Phys. Chem. A 94, 240 (2020).
S. S. Sologubov, A. V. Markin, Yu. A. Sarmini, et al., J. Chem. Thermodyn. 153, 106318 (2021).
S. A. Milenin, E. V. Selezneva, P. A. Tikhonov, et al., Polymers 13, 606 (2021).
J. Meija, T. B. Coplen, M. Berglund, et al., Pure Appl. Chem. 88, 265 (2016).
V. M. Malyshev, G. A. Mil’ner, E. L. Sorkin, et al., Prib. Tekh. Eksp., No. 6, 195 (1985).
R. M. Varushchenko, A. I. Druzhinina, and E. L. Sorkin, J. Chem. Thermodyn. 29, 623 (1997).
R. Sabbah, A. Xu-wu, J. S. Chickos, et al., Thermochim Acta 331, 93 (1999).
G. W. H. Hohne, W. F. Hemminger, and H.-J. Flammersheim, Differential Scanning Calorimetry (Springer, Heidelberg, 2003).
P. Gabbott, Principles and Applications of Thermal Analysis (Blackwell, Oxford, UK, 2008).
G. della Gatta, M. J. Richardson, S. M. Sarge, et al., Pure Appl. Chem. 78, 1455 (2006).
E. Kaisersberger, J. Janoschek, and E. Wassmer, Thermochim. Acta 148, 499 (1989).
G. Adam and J. H. Gibbs, J. Chem. Phys. 43, 139 (1965).
W. Kauzmann, Chem. Rev. 43, 219 (1948).
A. B. Bestul and S. S. Chang, J. Chem. Phys. 40, 3731 (1964).
P. Debye, Ann. Phys. (N.Y.) 344, 789 (1912).
Experimental Thermodynamics, Vol. 1: Calorimetry of Non-Reacting Systems, Ed. by J. P. McCullough and D. W. Scott (Butterworth, London, 1968).
M. W. Chase, J. Phys. Chem. Ref. Data 1–2, 1951 (1998).
Funding
This work was supported by the RF Ministry of Science and Higher Education as part of State Task no. 0729-2020-0053; and by the Russian Foundation for Basic Research, project no. 19-03-00248.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare they have no conflicts of interest.
Additional information
Translated by K. Utegenov
Rights and permissions
About this article
Cite this article
Smirnova, N.N., Markin, A.V., Sologubov, S.S. et al. Thermodynamic Properties of a Fourth-Generation Carbosilane Dendrimer with Terminal Trimethylsilylsiloxane Groups. Russ. J. Phys. Chem. 96, 1637–1646 (2022). https://doi.org/10.1134/S0036024422080210
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024422080210