Abstract
The thermal stability of pure urea–formaldehyde resin (PR) and modified urea–formaldehyde (UF) resins with hexamethylenetetramine-HMTA (Resin 1), melamine-M (Resin 2), and ethylene urea (EU, Resin 3) including nano-SiO2 was investigated by non-isothermal thermo-gravimetric analysis (TG), differential thermal gravimetry (DTG), and differential thermal analysis (DTA) supported by data from IR spectroscopy. Possibility of combining inorganic filler in a form of silicon dioxide with UF resins was found investigated and percentage of free formaldehyde was determined. The shift of DTG peaks to a high temperature indicates the increase of thermal stability of modified UF resin with EU (Resin 3) which is confirmed by data obtained from the FTIR study. The minimum percentage (6%) of free formaldehyde was obtained in Resin 3.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urea–formaldehyde resins are the most important type of the so-called amino plastic resins. Amino resins are often used to modify properties of other materials. These resins are added during the processing of products, such as textile fabrics to impart permanent press characteristics; automobile tires to improve the bonding of rubber to the tire cord; paper to improve its tear strength, especially of wet paper; and alkyds and acrylics to improve their curing properties. Amino resins are also used for molding products, such as electrical devices, jar caps, buttons and dinnerware, and in the production of countertops. As a typical amino resin, UF resin adhesive possesses some advantages, such as fast curing, good performance in the panel, water solubility, and lower price [1]. The formaldehyde emission from the panel used interior applications was one of the factors affecting sick building syndrome in an indoor environment. Therefore, the formaldehyde emission from UF resin-bonded wood product in air is a mayor problem in the particleboard and plywood industry [2].
Siimer et al. [3] examined the impact of content melamine on melamine–urea–formaldehyde resins structure using TG-DTA and 13C NMR spectroscopic analysis. The presence of triazine ring in their structure provides improved hydrolytic and thermal stability, compared with UF resin. Slonim et al. [4] using 13C NMR spectroscopic analysis examined the influence of NH3 and HMTA at UF resin structure which creates triazinone rings stabilized by methylol groups linked to amine nitrogen of the ring. In a UF system, uronic ring formation takes place in an acid or alkaline medium with a large excess of formaldehyde in the system.
Polymer nano-composites consisting of polymer and inorganic nano-materials have good advantages in thermal properties. Fillers have a modifying effect on the properties of UF resin. But the fillers formerly used are all particles with sizes above micron grade, which have only small modifying effect. Chemical activities of nanoparticles are excellent [5]. Given this property, our test introduces nanometer silicon dioxide (nano-SiO2), to be added to modified UF resin, which obtains favorable modifying results. For example, when used as composite fillers, silica materials (quartz, fumed silica, precipitated silica, colloidal silica, etc.) drastically improve the mechanical, thermal and rheological properties of the polymer matrix composites. Introduction of silica particle into UF adhesive substantially decreases the formaldehyde release into the environment. Silica particles strongly sorbs up to 15 wt% of formaldehyde released in curing of UF resin [6]. The surface hydroxyl groups of silica including isolated, vicinal, and geminal silanols are believed to play a key role in most of the aforementioned applications [7].
The effect of nano-SiO2 content with coupling agent on SiO2/UF resin was discussed by Qiaojia et al. [6]. The mechanism of the strengthening effects of nano-SiO2 on UF resin by means of infrared spectrum analysis and X-ray photoelectronic spectrum (XPS) was analyzed.
In this study, the thermal stability of pure urea–formaldehyde resin (PR) and modified UF resins with hexamethylenetetramine (HMTA, Resin 1), melamine (M, Resin 2), and ethylene urea (EU, Resin 3) including nano-SiO2 was investigated by non-isothermal thermo-gravimetric analysis (TG), differential thermal gravimetry (DTG), and differential thermal analysis (DTA) supported by data from IR spectroscopy. Possibility of combining inorganic filler in a form of silicon dioxide with UF resins was found investigated and percentage of free formaldehyde was determined.
Experimental
Materials
The following materials were employed in the study reported here.
Urea—(NH2)2CO (Alkaloid-Skoplje), 35% formaldehyde—CH2O (Unis-Goražde), hexamethylenetetramine—HMTA, C6H12N4 (Merck-Darmstadt), melamine—M, C3H6N6 (Merck-Darmstadt), ethylene urea—EU, (CH2)2(NH)2CO (Fluka-Buchs), and silicon oxide—SiO2 (Merck-Darmstadt) with specific surface 200 ± 25 m2/g.
Synthesis of modified UF resins
Three types of modified UF resins with formaldehyde to urea (F/U) ratio (0.8) were synthesized using the same procedure. Synthesis procedure of UF resins was shown as follows: 60 cm3 of distilled water and 0.1 mol of urea are mixed into reaction vessel with magnetic stirrer. Then, 0.015 mol of HMTA (Resin 1), M (Resin 2), and EU (Resin 3) are added. Other components such as 7.25 g SiO2, 0.12 mol 35% formaldehyde, and 0.6 cm3 of concentrated sulfuric acid were added into the reaction mixture according to following order. The pH values were determined: 2 for pure (PR) and Resin 1 (HMTA), 1.5 for Resin 2 (M) and 1.5 for the Resin 3 (EU), respectively. Reaction mixture was mixed for 3 h. 0.22 mol of sodium hydroxide dissolved in 6 cm3 of distilled water and added to reaction mixture before the stirring was done. The pH value of each resin was: 3.5 for PR, 6.5 for Resin 1 (HMTA), 12 for Resin 2 (M), and 7.5 for the Resin 3 (EU), respectively. The modified UF resin is cured at 110 °C for 3 h in a convective drying oven. The contents of dry solid of each resin was: 39.7% for PR, 44.41% for Resin 1 (HMTA), 38.91% for Resin 2 (M), and 62.46% for the Resin 3 (EU), respectively.
Thermal stability of modified UF resins
Thermal analysis was carried out with a Perkin Elmer model 951 with digital programmer Du Pont Thermal analyzer model 1090. Samples (above 8 mg) were placed in alumina crucibles. An empty alumina crucible was used as reference. Samples were heated from ambient temperature to 600 °C in a 50 mL min−1 flow on N2. A heating rate of 10 °C min−1 was used and continuous record of sample temperature, sample mass, its first derivative and heat flow were taken.
Infrared spectrum analysis
The FTIR spectroscopy (Perkin-Elmer Fourier-transform infrared spectroscope-FTIR series 1600), in transmittance mode, was used for the characterization of the functional group of the resin. For the solid samples, KBr pellets with 1 mass% of the powdered material were produced. The spectra were obtained in the spectral area 4000–500 cm−1, with a resolution of 2 cm−1.
Free formaldehyde determination
Fifty cubic centimeters of 1 mol of pure sodium sulfite solution was prepared in 500-cm3 flask. Three drops of thymol phthalein indicator were added. The mixture was carefully neutralized by titration with 1 M hydrochloric acid until the blue color of the indicator disappeared. 0.5 g of the resin sample was added to the 25 cm3 of destiled water and 15 cm3 of 0.5 mol/dm3 sodium sulfate solution. A few drops of thymol phthalein indicator were added and the resulting mixture was titrated with 0.5 mol/dm3 hydrochloric acid until complete decolorization was obtained. The experiment was carried out once at the end of the condensation reaction of urea and formaldehyde.
The free formaldehyde CH2O (%) content was calculated from the Eq. 1 given below.
where V is the volume of HCl (cm3), c is the concentration of HCl (mol/dm3), E is the equivalent weight of formaldehyde, and a is the weight of samples (g).
Results and discussion
Urea–formaldehyde (UF) resins are thermosetting polymers, prepared by the reaction of two monomers, urea and formaldehyde. This reaction is basically a two-step process: usually an alkaline methylolation followed by an acid condensation. Methylolation refers to the addition of up to three molecules of urea of the bifunctional formaldehyde to one molecule of urea to give the so-called methyloureas. This reaction present a series of reactions that lead to the formation of mono-, di- and trimethylolureas (Scheme 1)
The UF polymer builds up in the acid condensation stage. The methylols, urea and formaldehyde is still present in the system, reacting to give a linear and partly branched molecules with medium and even higher molar masses [8, 9]. The type of bond between the urea molecules depends on the reaction conditions: low temperatures and only slightly acid pH favor the formation of methylene ether bridges (–CH2–O–CH2), while higher temperatures and lower pH lead to the stable methylene (–CH2–) bonds:
Ether bridges can rearrange to methylene bonds by splitting off formaldehyde [10]. After hardening UF resin form an insoluble, three-dimensional network and cannot be melted or thermoformed again. By using different conditions of reaction and preparation more or less innumerable variety of condensed structures is possible.
In the studies of many authors is has been pointed out that formaldehyde is mainly emitted from the following sources: residual formaldehyde in the resin; formaldehyde generated by condensation reactions between hydroximethyl groups and formaldehyde released by hydrolytic degradation of weakly bonded structures is cured resin [2] (Scheme 2c, d).
Thermal stability of the modified UF resin
During UF resin manufacturing, the final reaction products between urea and formaldehyde can range from the simple monomethylolurea to very complicated cross-linked structures. During resin curing, a three-dimensional network structure is built up. The formation of linear condensation products in cure process begins at lower temperature, if the resin contains greater amount of reactive methylol groups [11]. Depending on different synthesis conditions and technology, the generally used two-step reaction of urea and formaldehyde produces resins with a broad variety of both linear and branched chains.
The thermal behavior of modified UF resins occurs generally in three main stages (Fig. 1; Table 1). The first-step degradation occurs in the temperature region of 32.2–107, 55.9–217.7, 51.6–185.2, and 76.4–209.2 °C for pure and Resins 1, 2, and 3, respectively, and the mass loss is range to 7.1, 6.2, 4.5, and 4.9% most indicating the evaporation of water. Above 200 °C radicals formed by chain scission induce the formation of cyclic structures in the polymer which undergoes extensive fragmentation above 300 °C [12].
The second-step degradation starts at 223 °C and ends at 243.9, 236–494.6, 202.8–403.5, and at 226–459.3 °C for pure and Resin 1, 2, and 3, respectively. The percent of mass loss at second-step degradation is 12.3, 37.1, 34.4, and 31.6, indicating polymer degradation. The percentage of pure resin’s mass loss is three times smaller than in modified resins which are show consistent mass loss. This is confirmed by the DTG analysis. Two degradation processes are overlap and they are continues from 252.3 °C with mass loss of 58.5%.
Third-step degradation starts at 508.1, 423, 478, and ends at 600 °C, for resin with HMTA, M, and EU, respectively. The percent of mass loss at third-step degradation is 8.8, 20.5, and 12.0 for modified UF resin with HMTA, M and EU, respectively.
Differences in mass loss of modified UF resins can be attributed to outgoing both of chemically bounded water from silica’s inside and triazine from silica surface in resin’s. This is confirmed by Kaminski et al. [13] investigations which are 11–14% mass loss measured during triazine-silica hybrid structure decomposition.
Table 1 presents the DTG and DTA peak values of modified UF resins. The shift of values of the DTG peaks to a high temperature indicating increased thermal stability modified UF resin with EU.
In Fig. 1, the curve TG (a), DTG (b) and DTA (c) of pure and modified UF resins with HMTA, M and EU are presented. It can be seen that DTA curve shows the endo- and exothermic peaks. The small endothermic peaks of water evaporation with a minimum at 82.5, 101.1, 80.4, and 98.8 °C are derived from initial resin water and from condensation reaction for pure resin and Resin 1, 2, and 3, respectively. After water evaporation up to a temperature of 248.7, 243.2, 211.2, and 265.5 °C there are no thermal effects for pure resin and Resin 1, 2 and 3, respectively.
Degradation process of the resins begins from 200 °C. The endothermic peaks with minimum at 279.1, 294.0, 263.3, and 315.1 °C is attributed by many authors to the degradation of methylene ether bridges into methylene bridges and branching and cross-linking reactions in the resins network [14]. Degradation process of cured resin begins with liberation of formaldehyde from dimethylene ether groups (Scheme 2c, d).
This kind of degradation process can be regarded as post curing of resin, as released formaldehyde participates in further reaction, finally giving more stable methylene group. This proves endothermic peak with a minimum at 292.5, 294.0, 263.3, and 315.1 °C for pure and resins 1, 2, and 3, respectively, belongs to decomposition of the most stable units in the UF resin-methylenediurea. The small endothermic peak for Resin 1, 2, and 3 with minimum at 342.1, 405.6, and 426.9 °C belong to dehydration of the silanol groups in amorphous silica particles causes small mass reduction. The large exothermic peak with maximum at 509.5, 520.2, and 513.1 °C was indicated which are contributed to water loss from the silica particles with modification of the structure. As the temperature increases further up to 600 °C, the DTA curve showed no peaks, and the TG curve is indicated a slow decrease in the sample mass, i.e., a loss of chemically bound water that is contained in hydroxyls in the form of single or double silanols groups in the SiO2 structure (Scheme 3).
Infrared spectrum analysis
The FTIR spectra of the pure and modified UF resin samples are shown in Fig. 2 and Table 2. The spectrum of the modified UF Resin 1 (Fig. 2) shows a strong adsorption band at 3467 cm−1. This band is sharp and typical of hydrogen bonded N–H and OH. However, when the reaction was conducted in acid medium the spectrum of the modified UF resins 1 and 3 in the 3467–3385 cm−1 region is sharper than of pure (3353 cm−1) and modified UF resin 2 (3307 cm−1). The sharpness of these bands were indicated a reduction in the extent of hydrogen bonded interaction which is expected as the structure becomes more cross-linked due to methylenization reaction [15].
The absorption frequencies are broad of resin spectra, due to complexity of polymer structure. Broadening may also be observed due to the presence of byproducts in the resin, such as water and excess formaldehyde, which allow hydrogen bonding with the reactive functional groups like –CH2OH, NH2, and –NH present in resin samples [16]. However, spectra of cured resins showed sharper characteristic absorption peaks in this region. A medium absorption peak of the pure UF resin is observed at 3353 cm−1, which is characteristic absorption of the NH-stretching mode for –NH2 group. Similar absorption bands for Resin 1, 2, and 3, were recorded in the same region. A weak absorption band in pure resin spectra appears in the range of 3040–2970 cm−1, which is ascribed to the symmetrical C–H stretching mode of CH2 of ether, CH2OH and N–CH2. A very strong absorption band is observed around ~1640, 1681, 1675, and 1682 cm−1 for pure and resin 1, 2, and 3, respectively, in the spectra, which may be assigned to the C=O stretching (amide-I) in –CONH2 group. The strong absorption bands around 1551, 1616, 1617 and 1598 cm−1 for pure and Resins 1, 2, and 3, respectively, may be due to –NH bending mode (amide-II) are assigned. The cross-linking between two methylol groups provide ether linkages (–CH2–O–CH2) to which –NH is attached on both the sides. The weak absorption bands at around 1350–1470 cm−1 for all polymer samples may be ascribed to C–H bending mode in –CH2/–CH2OH/N–CH2–N. The medium absorption band in the region of 1150–1130 cm−1 is appearing, which is assigned to asymmetric stretching vibrations of –N–CH2–N– group, ν(C–O–C) of ether linkage and ν(Si–O–C) and νass(Si–O–Si) of siloxane or silicone [17, 18]. Asymmetric streching vibrations of C–O in –CH2OH, N–H wagging in 1° and 2° amines, ν(Si–O) and triazinone rings at ~780–840 cm−1 is assigned, respectively [13]. At 608–706 cm−1 –CH bending mode is ascribed.
Crosslinking between organic molecules and surface of SiO2 results in the replacement of the most strongly hydrophilic silanol functionality with a material exhibiting modified. The most used proces of attachment of an organic moiety to silica surface involves formation of Si–O–Si or Si–O–C bonds which confirms the existence of absorbtion band at 1150–1130 cm−1.
Absorbtion bands assigned to the C=O stretching (amide-I) in –CONH2 group and –N–CH2–N– from –NH bending mode (amide-II) and νass(Si–O–Si) of siloxane or silicone respectibility in resin 3 are the most shifting to higher wave numbers. This is resulting as maximum number of interaction between macromolecule of resin and silica groups. This move is a result that Resin 3 has the most thermal stability in relation to Resin 1 and 2.
Free formaldehyde determination
Formaldehyde can be bound with sorbents compatible with UF polymer, in particular, with silicon dioxide having large specific surface area. Bonding between organic molecules and functional groups of silica is resulting replacement of most of the strongly hydrophilic silanol functionality with a material exhibiting modified, and usually predictable, new or improved properties [13]. The amount of free formaldehyde contained in the UF resin is reduced by its sorption by SiO2 particles [19].
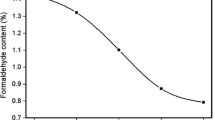
Table 3 gives the percentage-free formaldehyde content of the modified UF resin samples obtained from the three different UF resin. From Table 3, it can be clearly seen that the in Resin 1, 2, and 3 drastically reduced contents of the free formaldehyde. The percentage of free formaldehyde importance decreases from 15% for pure UF resin to 6% for Resin 3, with the percentage of free formaldehyde identical at Resin 1 and Resin 2 (8%).
Conclusions
TG/DTG and DTA curves show that the modified UF resins compared with pure resin have good thermal stability. The DTG peaks value is shifting to a high temperature indicating increased thermal stability of modified UF resin with EU (Resin 3).
Water evaporation from curing system exhibits by broadly small endothermic peak in DTA curve with a minimum at 82.5, 101.1, 80.4, and 98.8 °C for pure and Resin 1, 2, and 3, respectively. Due to evaporation of water from system, the resin molecules are less mobile and the polymeric constituents of the surface area of filler slow down the contact between reactive sites of the resin. Common to all resins is that the further heating up to 200 °C, causes no changes in the reaction heat values. At temperatures over 200 °C, the resins begin to decompose showing series of endothermic and exothermic effects in similar temperature regions.
The FTIR spectra (shape) of the modified UF resin samples depend on the pH environment. When the pH reaction decreased (acid medium) the characteristic absorption bands of the modified UF resins became sharper and move to lower values of wave number. Absorption bands assigned to the C=O stretching (amide-I) in –CONH2 group and –N–CH2–N– from –NH bending mode (amide-II) and νass(Si–O–Si) of siloxane or silicone respectibility in Resin 3 are the most shifting to higher wave numbers. This is resulting as maximum number of interaction between macromolecule of resin and silica groups. This move is a result that Resin 3 has the most thermal stability in relation to Resin 1 and 2. Resin 3 which includes the EU has the smallest percentage offree formaldehyde amount (6%).
References
Park BD, Lee SM, Roh JK. Effect of formaldehyde/urea mole ratio and melamine content on the hydrolytic stability of cured urea-melamine-formaldehyde resin. Eur J Wood Prod. 2009;67:121–3.
Siimer K, Kaljuvee T, Pehk T, Lasn I. Thermal behaviour of melamine-modified urea-formaldehyde resins. J Therm Anal Calorim. 2010;99:755–62.
Siimer K, Cristjanson P, Kaljuvee T, Pehk T, Lasn I, Saks I. TG-DTA study of melamine-urea-formaldehyde resins. J Therm Anal Calorim. 2008;92:19–27.
Slonim I, Alekseyeva SG, Urman YaG, Arshava BM, Aksel’rod BYa, Gurman IM. 13C-NMR determination of the structure on linear-branched urea-formaldehyde resins. Polym Sci. 1977;19:899–909.
Qiaojia L, Guidi Y, Jinghong L, Jiuping R. Property of nano-SiO2/urea formaldehyde resin. Front For China. 2006;2:230–7.
Dudkin BN, Krivoshapkin VP, Krivoshapkina EF. Effect of aluminium oxide nanoparticles on the properties of urea-formaldehyde resin. Russ J Appl Chem. 2006;79:1522–5.
Wan Q, Ramsey C, Baran G. Thermal pretreatment of silica composite filler materials. J Therm Anal Calorim. 2010;99:237–43.
Park BD, Kim YS, Singh AP, Lim KP. Reactivity, chemical structure, and molecular mobility of urea-formaldehyde adhesives synthesized under different condition using FTIR and solid-state 13C CP/MASS NMR spectroscopy. J Appl Polym Sci. 2003;88:2677–87.
Zorba T, Papadopoulou E, Hatjiissaak A, Paraskevopoulos KM, Crissafis K. Urea-formaldehyde resins characterised by thermal analysis and FTIR method. J Therm Anal Calorim. 2008;92:29–33.
Conner HA. Urea-formaldehyde adhesive resins. In: Joseph C, Salamone JC, Demby A, Aller M, editors. Encyclopedia of polymeric materials, vol. 2. Boca Raton: CRC; 1996. p. 8495–500.
Siimer K, Kaljuvee T, Christjanson P, Lasn I. Curing of Urea-formaldehyde resins on a wood substrate. J Therm Anal Calorim. 2006;84:71–7.
Camino G, Operti L, Trossarelli L. Mechanism of thermal degradation of urea-formaldehyde polycondensates. Polym Degrad Stab. 1983;5:161–72.
Kaminski Z, Mizerski M, Kolesinska J, Psarski M, Kuberski S, Plaza S. Synthesis and microtribological studes of new silica-triazine hybrid. Tribol Lett. 2006;22:119–25.
Siimer K, Kajuvee T, Christjanson P. Thermal behaviour of urea-formaldehyde resin during curing. J Therm Anal Calorim. 2003;72:607–17.
Edoga MO. Comparative study of synthesis procedures for urea-formaldehyde resins (Part I). Leonardo Elect J Pract Tehnol. 2006;9:63–80.
Dipak K, Raval, Bhavil N, Narola, Amit J, Patel AJ. Synthesis,characterization and composites from resorcinol-urea-formaldehyde-casein resin. Iran Polym J. 2005;14(9):775–84.
Bailey RJ, McGuire MM. ATR-FTIR Observations of water structure in colloidal silica:Implication for the hydratation force mechanism. Langmuir. 2007;23:10995–9.
Farhadyar N, Rahimi A, Langroudi AE. Preparation and characterization of aromatic amine cured epoxy-silica hybrid inorganic-organic coating via in situ sol-gel process. Iran Polym J. 2005;14:155–62.
Leonovich AA, Kovrizhnykh LP, Korneev VI, Bodoyavlenskaya GA, Medvedeva IN. Silicondioxide as a component of urea-formaldehyde adhesive. Russ J Appl Chem. 2002;75:1336–8.
Acknowledgements
Authors acknowledge the support of the Ministry of Science of the Republic of Serbia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Samaržija-Jovanović, S., Jovanović, V., Konstantinović, S. et al. Thermal behavior of modified urea–formaldehyde resins. J Therm Anal Calorim 104, 1159–1166 (2011). https://doi.org/10.1007/s10973-010-1143-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-1143-8