Abstract
The decomposition of series of supramolecular compounds, namely inclusion compounds, was studied by means of different thermoanalytical methods, i.e., traditional thermogravimetry, quasi-equilibrium thermogravimetry, and thermomechanical analysis. The series of compounds included the intercalates on the base of fluorinated graphite C2F, the clathrates on the base of carbamide and on the base of coordination compounds and microporous inclusion compounds on the base of coordination compounds. Kinetic parameters of decomposition processes were estimated within the approaches of the non-isothermal kinetics (“model-free” kinetics, linear and non-linear regression methods for the topochemical equation detection). The kinetic stability of the inclusion compounds under heating, the flexibility of the matrix structure, and the thermodynamic stability of the intermediate phases are discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Supramolecular chemistry deals with the associates of two or more chemical species, which held together by weak chemical bonds such as intermolecular Van-der-Waals interactions, or others. This science field includes special subjects such as molecular recognition, molecular complementarity, self-assembling, and crystal engineering [1].
The term molecular recognition refers to the specific interaction between two or more molecules through non-covalent bonding. Static molecular recognition is the interaction between a key and a keyhole, a reaction between a host matrix and a guest molecule to form a host–guest complex. The “inclusion compounds” results when the host provides a hollow space, or precisely defined cavity. The host matrix and the guest molecules, involved in such molecular recognition, have molecular complementarity.
One of the important aspects of thermodynamic stability is the intermediate phase’s stability during the thermal decomposition. The method of quasi-isobaric and quasi-isothermal thermogravimetry [2] can be realized as quasi-equilibrium thermogravimetry for the decomposition processes with high reversibility and without diffusion hindrance. The decomposition of inclusion compounds is that process.
The existence of any compound at the room temperature (under air storage) means that the substance is stable with respect to all possible decomposition reactions: dehydration, dehydroxylation, deintercalation, redox reactions, pyrolysis, etc. This means that the Gibbs potential is negative (ΔG < 0), and (or) the rate constant of decomposition is small enough: the compound is thermodynamically and kinetically stable at room temperature and air pressure.
Experimental
Thermal analysis
TG measurements in quasi-isobaric, quasi-isothermal conditions [2] were carried out on a Derivatograph Q–1500–D (MOM, Hungary). The experiments were performed in air atmosphere, at constant decomposition rate 0.4 mg min–1, the sample mass was kept 200–500 mg, with different types of sample holders.
TG measurements for the kinetic studies were carried out on a Netzsch thermal analyzer TG 209 F1. The experiments were performed in argon flow, at constant heating rates from 4 till 20 K min−1, the sample mass was kept cca 12.0–15.0 mg. Thermomechanical analysis was carried out on a Netzsch thermal analyzer TMA 202; the powder layer thickness was 1.00–1.03 mm (with sample mass 42–45 mg).
Kinetic analysis under non-isothermal conditions
Thermogravimetric data were processed with the computer program Netzsch Thermokinetics 2 (Version 2004.05). Special program module “Model free”, based on the papers [3–11], allows processing several thermogravimetric curves, obtained with different heating rates, and calculating energy of activation, without the preliminary information about the kinetic topochemical equations. The programs “Ozawa–Flynn–Wall Analysis” and “Friedman Analysis” were used for the calculation of activation energies for the every experimental point of fractional conversion (in the interval 0.005 < α < 0.995).
The same set of experimental data was used further for searching the topochemical equation (the selection was done from 16 equations: chemical reaction on the interface, nucleation, and diffusion). This calculation is made by the improved multiple linear regression method [11].
The F test is used for the search of the best kinetic description, for the statistical control of the obtained equation. It tests the residual variances of the individual models with the aim to find whether the models differ significantly (statistically speaking). If F exp(1) ≈ F exp(2) for two equations, there is no reason to assume that the first model is better for the characterization of the experiment. The statistical quantile, F crit, is obtained for a level of significance = 0.05 [11].
The special program of multivariate non-linear regression is useful in search of the full set of kinetic parameters for multi-step processes. The random error in activation energy values for such reversible decomposition reaction under such experiments usually is about 10%, and we took this into the consideration.
The “Model free” approach and the work with linear regression and non-linear regression programs were widely discussed in references [3–11].
All details of the compounds synthesis, the conditions of the thermoanalytical experiment itself and full set of obtained kinetic data were published elsewhere. Here we discuss in broad terms general regularities in thermodynamic and kinetic stability of supramolecular compounds on the base of data published previously [12–17].
Results and discussion
Thermodynamic stability of the intermediate phases
We studied the decomposition of the clathrates, inclusion compounds, where organic amines (such as pyridine or 4-metyl-pyridine) are both the ligands in the host matrix, and the extra molecules of guests: [MPy4(NO3)2] · 2Py and [M(4-MePy)4(NCS)2] · 0.67(4-MePy) (M=Mg, Mn, Co, Fe, Ni, Cu, Zn) [12]. The synthesis is simple: the interaction between the liquid amine and metal salts.
By means of quasi-equilibrium thermogravimetry we can check all intermediate thermodynamically stable phases; the steps are well differentiated: the loss of methyl-pyridine molecules, one by one: guest molecules at the beginning, ligands from the host matrix later (Fig. 1). But for inclusion compounds of cobalt, nickel, and zink there is no stable host matrix because of the simultaneous loss of three pyridine molecules (Fig. 2); only the copper forms the stable host matrix irrespectively of guest presence.
The explanation is the absence of the stable “empty” host matrix phase [ZnPy4(NO3)2] in the system “zinc nitrate–liquid pyridine”; thermodynamically stable phases are [ZnPy3(NO3)2] and [ZnPy4(NO3)2] · 2Py. Such behavior of the system was called “the contact stabilization” of the host matrix structure during clathrate formation [12]. The inclusion compound, with matrix and guest molecules, is formed as a whole, but the empty host structure does not exist at all.
The quasi-equilibrium thermogravimetry is useful for the study of layered inclusion compounds such as intercalates. These inclusion compounds with the fluorinated graphite matrix C2F are usually synthesized as the intercalates of the first stage of filling: one layer of the matrix–one layer of guest molecules [13]. They lose a half of the guest molecules very easily and transform to the second stage of filling (two layers of the matrix–one layer of guest molecules)—quite stable on the air at the room temperature.
The intercalates of the fluorinated graphite with some guest molecules (such as chloroform and benzene) can be synthesized not with monomolecular layers only, but with the double layers of guest molecules [14]. These intercalates with two layers of guest molecules are stable only under excess of liquid guest. We used “wet” sample for the thermoanalytical study, the liquid guest excess evaporates at the beginning of the heating, and later there is the step of solid intercalate decomposition. The initial quantity of liquid chloroform is unknown, but the obtained stable phase at 350 K is the second stage of filling (Fig. 3). So we can recalculate the mass loss of this step (Δm ≈ 30% between two solid phases).
This calculation shows that the excessive layer of guest molecules removes together with the surplus of liquid chloroform or benzene, with the formation of the thermodynamically stable first stage of filling (one layer of the matrix–one layer of guest molecules). The phase with the double filling by the guest is thermodynamically unstable. The next mass loss is the transformation from the first stage of filling to the second stage of filling. These steps are observed only in the labyrinth holder and in the crucible with lid, i.e., under high partial pressure of guest molecules.
Kinetic stability of inclusion compounds
There are several important assumptions and limitations [15–17]:
The powders of compounds consist of microcrystals. The chemical reaction of decomposition takes place in the reaction zone, either on the grain surface, or in the bulk of this grain. We know the detailed crystal structure, but this reaction zone has not the regular crystal structure; the interplanar spacings are increased, lengths of chemical bonds are expanded, and the whole reaction zone structure is disordered. Therefore, it is not correct to search the direct connection between the known crystal structure and the kinetic parameters of decomposition.
The kinetic equations, used for the calculation of the kinetic parameters for the decomposition of solids, are topochemical ones [18–20].
We use the rate equation in the form V = k rate · f(α), where α is the degree of conversion. The rate constant can be expressed by the Arrhenius equation
There is the generalized form of the conversion function, i.e., the Sestak–Berggren equation [18]:
All specific equations can be formally built up from this one.
The calculations in the non-isothermal kinetics include the so-called single step approximation, which can not be valid for all cases [21, 22].
From the point of view of the classical chemistry of solids these kinetic parameters (E a, A) are not connected with the elementary act, the activated complex size and configuration are unknown, so the calculated kinetic parameters for the single compound are apparent ones and have limited physical meaning. For example the calculation of ∆H* and ∆S* from these parameters is the mistake.
Very important is the general trend in the variation of these values within a specially selected series of compounds: or isostructural compounds, or genetic connected compounds, because the expected disorder in the reaction zones can be identical for them [16, 17, 23, 24].
Among such compounds series are inclusion compounds: or with one host matrix and different guest molecules, or with different host matrices and one and the same guest.
The evaluation of the stability of inclusion compounds under heating
The existence of the compound itself is based on the stability in any processes of decomposition at room temperature and pressure. For inclusion compounds the possibility of the breakdown to the unfilled matrix and guest molecules is very important.
The peculiar properties of the “thermal stability” of inclusion compounds are discussed in the reference, and the difference between the onset temperature of compound decomposition and the boiling point of the guest (T on − T b) is recommended as the measure of the relative stability of an inclusion compound [25].
We studied the decomposition of such a series: a given host with the variety of guests and considered the importance of the boiling point of the guest.
Traditional clathrates
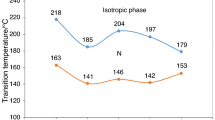
The kinetic data for the thermal decomposition for clathrates compounds of carbamide with alkanes NH2(CO)NH2 · yC n H2n+2 are presented in Table 1 [26]. Onset temperatures and kinetic parameters are non-monotonous (Fig. 4); there are two series: for even alkanes and for odd alkanes.
In both series the onset temperature increases, the activation energies decreases, the pre-exponential factor decreases. The increase of so called “thermal stability” (that is the onset temperature, T on) is defined by the pre-exponential factor decrease—that is by the entropy factor, not by the guest boiling temperature.
As to pure alkane properties, they form one series on boiling temperatures, but different series on melting temperatures (Table 1) [27]. It is connected with different molecules packing in the solid alkanes structures: “head-to-head” or “head-to-tail”.
We obtain the non-monotonous, two-series dependence of kinetic stability of inclusion compounds with “even” and “odd” alkanes (Table 1; Fig. 4). So the alkane guest molecules within the intercalate phases have “solid” properties; they “sublimate” from the solid. There’s no point speculating about the “boiling temperature” in consideration of the stability comparison (so as water boiling temperature and onset temperature of dehydration for the crystalline hydrate).
The best way for any physicochemical evaluation and comparison of the inclusion compounds stability is the calculation of the kinetic parameters of decomposition.
We studied the kinetics of the decomposition reaction for several series of inclusion compounds, where the host matrix is the coordination compound.
There is the set of kinetic parameters of the decomposition for inclusion compounds with different host matrices (Fig. 5) [28]:
The host matrix in the inclusion compound is stable only with the guest molecules support. The series with the increase of stability is Cu < Cd < Mn < Co. It is interesting that the kinetic stability is connected with the length (and therefore with the strength) of metal–nitrogen bonds in the coordination sphere of host matrix [29, 30]; bond distances (M–N) within the coordination sphere [M(Me–Py)4(NCS)2] are:
Therefore, the breakup of these inclusion compounds under heating depends on the coordination sphere (host matrix) stability, but not on the guest molecules being removed from the rigid matrix.
Microporous inclusion compounds
[31]. We study the inclusion compounds on the base of manganese formate matrix with tetrahydrofuran and dioxane guest molecules (five- and six-member cycles).
The onset temperature of the decomposition T on for the compound with dioxane is ≈450 K (Fig. 6, heating rate 4 K min−1). The inclusion compound with tetrahydrofuran captured the water molecule and loses it at the beginning of the decomposition (heating rate 4 K min−1; T on ≈ 370 K); we used for the calculation the part of the curve after the dehydration (T on ≈ 400 K) [31].
The mass loss of the organic guest molecules is two-staged; it is possible that this is connected with the existence of several structure modification of pure zink formate. It is important that both compounds are stable until 370 K (with tetrahydrofuran) and 450 K (with dioxane) at heating rate 4 K min−1.
The “model free” approach gives constant activation energy (with usual error, less than 10 percent) for the most part of decomposition reactions, the best equations was selected by the linear and by non–linear regression methods. There are the results [31].
The host structure behavior is different (Fig. 7). The smaller tetrahydrofuran molecules leave the matrix practically without its expansion, and the empty host matrix collapses during the decomposition at 370–410 K. Huge dioxane molecules leave the compound in a different way: the compound structure begins to expand from the very beginning of the heating (the area without the mass loss), the expansion is in progress until the beginning of decomposition (450 K). This expanded empty matrix collapses above 470 K. We think that such difference in decomposing structures results in different topochemical equations: the equation of the contracting sphere in the first case and the equation of the autocatalytic reaction in the second case. The second inclusion compound expands under heating, but mass loss begins after 450 K only. After this the kinetic parameter values for small molecules removal from initial structure and for huge molecules removal from beforehand expanded structure are identical.
Conclusions
The difference between the onset temperature of compound decomposition and the boiling point of the guest (T on − T b) was recommended as the useful measure of the relative thermal stability of an inclusion compound [25].
But the onset temperature can be connected not only with activation energy values, but with the entropy factor (as for the clathrates with alkanes) [26]. The difference between the onset temperature (kinetic parameter) of the inclusion compound decomposition and the boiling temperature (thermodynamic parameter) of the guest has no physicochemical meaning.
The supramolecular compounds are broadly studied, but special investigations of the stability under heating are very rare, and they have no quantitative data on the kinetic stability [32, 33].
Up-to-date thermoanalytical instruments and up-to-date computer programs for experimental data processing give the possibility to study both thermodynamic and kinetic stability of supramolecular compounds in the processes of thermal decomposition.
The important thermodynamic information includes the composition of stable intermediate phases and the enthalpy and entropy values. The important kinetic information includes the onset temperatures, calculated kinetic parameters, and the shift of the values of the energies of activation and pre-exponential factors in the series, the change in the compound structural volume.
The more simple approach, connected with the “thermal stability” evaluation, based only on the comparison of the initial temperature of decomposition of inclusion compounds is not successful enough for the meaningful interpretation of the decomposition processes.
The layered inclusion compounds on the base of C2F matrix have one peculiar behavior: the high stability of the host matrix itself and the easy exchange of the guest molecules. The kinetic stability of inclusion compounds, based on the coordination compounds matrices, depends often on the host structure stability [12, 31, 34]. The topochemical equation and kinetic parameter values (in the compounds series) can be connected with the structure response on the heating and can depend on the flexibility of the matrix structure [13, 24]. The clearing-up of the regularities between the host structure and the whole substance stability is important for the chemistry of supramolecular compounds.
References
Atwood JL, Steed JW, editors. Encyclopedia of supramolecular chemistry. Boca Raton: CRC Press Science; 2004.
Paulik J, Paulik F. Simultaneous thermoanalytical examinations by means of the derivatography. New York: Elsevier Scientific Publishing Company; 1981.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Friedman HL. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. J Polym Sci C. 1963;6:183–95.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Ozawa T. Estimation of activation energy by isoconversion methods. Thermochim Acta. 1992;203(C):159–65.
Flynn JH, Wall LA. General treatment of the thermogravimetry of polymers. J Res Nat Bur Stand. 1966;70:478–523.
Opfermann J, Kaisersberger E. An advantageous variant of the Ozawa–Flynn–Wall analysis. Thermochim Acta. 1966;203(C):167–75.
Opfermann JR, Kaisersberger E, Flammersheim HJ. Model-free analysis of thermo- analytical data-advantages and limitations. Thermochim Acta. 2002;391:119–27.
Vyazovkin S. Model-free kinetics: staying free of multiplying entities without necessity. J Therm Anal Calorim. 2006;83:45–51.
Netzsch Thermokinetics 2. Version 2004.05; http://www.therm-soft.com.
Dyadin YA, Soldatov DV, Logvinenko VA, Lipkowski J. Contact stabilization of host complex molecules during clathrate formation: the pyridine-zinc nitrate and the pyridine-cadmium nitrate systems. J Coord Chem. 1996;37:63–75.
Pinakov DV, Logvinenko VA. The relationship between properties of fluorinated graphite intercalates and matrix composition. Intercalate with acetonitrile. J Therm Anal Calorim. 2006;86:173–8.
Yudanov NF, Chernyavski LI. The model of the structure for the intercalates on the base of the fluorinated graphite. Zhurn Struct Khimii. 1987; 28:86–95 (in Russian).
Logvinenko VA. Thermoanalytical approach to the study of the kinetic and thermodynamic stability of coordination compounds and clathrates. J Therm Anal. 1990;36:1973–80.
Logvinenko V. Stability and reactivity of coordination and inclusion compounds in the reversible processes of thermal dissociation. Thermochim Acta. 1999;340–1:293–9.
Logvinenko V. Solid state coordination chemistry. The quantitative thermoanalytical study of thermal dissociation reactions. J Therm Anal Calorim. 2000;60:9–15.
Sestak J, Berggren G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim Acta. 1971;3:1–12.
Sestak J. Thermophysical properties of solids. Their measurements and theoretical thermal analysis. Prague: Academia Prague; 1984.
Sestak J. Heat, thermal analysis and society. Pardubice: Nucleus NK; 2004.
Simon P. The single-step approximation: attributes, strong and weak sides. J Therm Anal Calorim. 2007;88:709–15.
Simon P. Single-step kinetics approximation employing non-arrhenius temperature functions. J Therm Anal Calorim. 2005;79:703–8.
Logvinenko V, Fedorov V, Mironov Y, Drebushchak V. Kinetic and thermodynamic stability of cluster compounds under heating. J Therm Anal. 2007;88:687–92.
Logvinenko V, Drebushchak V, Pinakov D, Chekhova G. Thermodynamic and kinetic stability of inclusion compounds under heating. J Therm Anal. 2007;90:23–30.
Nassimbeny LR. Physicochemical aspects of host-guest compounds. Acc Chem Res. 2003;36:631–7.
Logvinenko VA, Gegola OV, Chekhova GN, Dyadin YA. Kinetics of the thermal decomposition of clathrates of carbamide with n-alkanes. Conference on kinetics and mechanism of chemical reactions in solids. Abstracts, vol 1. Novosibirsk; 1977. p. 150–3 (in Russian).
Hauptmann S, Graefe J, Remane H. Lehrbuch der organischen chemie. Leipzig: VEB Deutscher Verlag fur Grundstoffindustrie; 1976.
Logvinenko VA, Soldatov DV. Processes of thermal dissociation of clathrates on the base of coordination compounds. J Therm Anal Calorim. 1999;56:485–92.
Soldatov DV, Logvinenko VA, Dyadin YA. The clathrates formation and phase equilibrium in the system Py–Zn(NO3)2. Zhurn Neorg Khimii. 1995;40:324–8 (in Russian).
Soldatov DV, Ukraintseva EA, Logvinenko VA, Dyadin YA, Grachev EV, Manakov AY. Thermodynamic dissociation constants for [MPy4(NO3)2]·2Py clathrates (M=Mn, Co, Ni, Cu). Supramol Chem. 2000;12:237–46.
Logvinenko V, Dybtsev D, Fedin V, Drebushchak V, Yutkin M. The stability of inclusion compounds under heating. Part I. Inclusion compounds of microporous manganese formate with included dioxane and tetrahydrofuran. J Therm Anal Calorim. 2007;90:463–7.
Margit B, Bombicz P, Madarász J. Thermal stability and structure of a new co-crystal of theophylline formed with phthalic acid TG/DTA-EGA-MS and TG-EGA-FTIR study. J Therm Anal Calorim. 2009;95:895–901.
Terekhova IV, De Lisi R, Lazzara G, Milioto S, Muratore N. Volume and heat capacity studies to evidence interactions between cyclodextrins and nicotinic acid in water. J Therm Anal Calorim. 2008;92:285–90.
Logvinenko V, Dybtsev D, Fedin V, Drebushchak V, Yutkin M. The stability of inclusion compounds under heating. Part 2. Inclusion compounds of layered zinc camphorate, linked by linear N-donor ligands. J Therm Anal Calorim. 2010;100: 183–9. doi: 10.1007/s1097300904442.
Acknowledgements
Author is grateful to Netzsch Geraetebau GmbH for the possibility to work with computer program “NETZSCH Thermokinetics 2” and RFBR for the financial support (Grants 07-03-00436 and 07-03-91208).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Logvinenko, V. Stability of supramolecular compounds under heating. J Therm Anal Calorim 101, 577–583 (2010). https://doi.org/10.1007/s10973-010-0900-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-0900-z