Abstract
A new, previously unknown phase Al8V10W16O85 has been obtained from reaction taking place in the solid state. It forms continuous solid solution with Fe8V10W16O85 of the Fe8−x Al x V10W16O85 general formula. All these phases are isostructural with M–Nb2O5 and (W0.35V0.65)2O5 and belong to a block structure phases with ReO3 type blocks of 4 × 4×∞ dimensions. Al8V10W16O85 is tetragonal and has the lattice constants a = b = 1.9487(1) nm and c = 0.36706(4) nm. It melts incongruently at 1,183 K depositing Al2(WO4)3 and WO3. The increase of the Al3+ ions content in the crystal lattice of Fe8V10W16O85 causes the melting point increasing, and decreasing of a = b unit cell parameters with c being almost constant. IR spectra of Al8V10W16O85 and Fe8−x Al x V10W16O85 phases have been recorded.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The oxidative dehydrogenation (ODH) of light hydrocarbons and selective reduction of NO with NH3 have been extensively studied over alumina or titania supported vanadium oxide catalysts [1–4]. A major challenge in the light hydrocarbons ODH catalytic technology is improvement in the alkene yields, because significant carbon oxide byproducts are also formed. One method to improve the yield of the desired product is to use metal oxide additives. The vanadium containing catalysts can be tuned with second or third component like Mo, W, Sb, Ni, and Co [1–4]. The effect of the incorporation of second or third component to V-containing catalysts results in a replace of the polyvanadate structure with less reactive V–O–X structure, leading to lower reducibility and oxidative dehydrogenation rates. In spite of extensive scientific studies on catalytic activity of multicomponent oxide systems, the knowledge on phase equilibria being established in an appropriate ternary or quaternary systems is unsatisfactory. In the case of Al2O3–V2O5–WO3 only binary systems have been the subject of investigations. In the Al2O3–WO3 system a phase with general formula Al2(WO4)3 forms, crystallizing in an orthorhombic system and melting congruently at 1,527 K [5, 6]. Whereas in the Al2O3–V2O5 system triclinic AlVO4 forms, being isostructural with FeVO4 [7]. AlVO4 melts incongruently at 1,018 K with deposition of α-Al2O3 as a solid product [7]. Literature data pertaining V2O5–WO3 imply that only solid solution of the V2−x W x O5 type with a x < 0.07 [8] or x ≤ 0.15 [9] solubility limit is formed. On the other hand, the Fe2O3–V2O5–WO3 system has been the subject of extensive studies [10–16]. The components of this system react with each other to form Fe8V10W16O85 phase [10–12]. Fe8V10W16O85 crystallizes in a tetragonal system [13], but its structure is unknown. This compound melts at 1,103 K with depositing two solid products, i.e. Fe2WO6 and WO3 [10]. Besides, a solid solution of V2O5 in Fe2WO6 has been found to occur in the three-component system [11]. It is also known that in the quaternary system Fe2O3–V2O5–WO3–MoO3 Fe8V10W16−x Mo x O85 solid solution is formed (for x ≤ 4), isostructural with Fe8V10W16O85 [14]. The equal charge and close values of Fe3+ and Al3+ ionic radii (R Al = 0.0535 nm and R Fe = 0.0645 nm in octahedral coordination), let one expect formation of solid solutions as a result of substitution of aluminum for iron in the quaternary Fe2O3–Al2O3–V2O5–WO3 system. This study shows the experimental results of the substitution of aluminum for iron in the case of Fe8V10W16O85 compound.
Experimental
The following materials were used for the research: V2O5, p.a. (POCh, Poland), WO3, 99.9% (Fluka AG, USA), Al2O3, pure (POCh, Poland), α-Fe2O3 p.a. (POCh, Poland), MoO3, pure (POCh, Poland), ZnO 99.9% (Sigma–Aldrich, Germany), and CoCO3, pure (POCh, Poland).
For the experiments five samples were selected with contents corresponding to Fe8−x Al x V10W16O85 formula with x = 0, 2, 4, 6, and 8. They represented the whole component concentration range of the system: Fe8V10W16O85–Al8V10W16O85. The content of V2O5 and WO3 in the all mixtures was always constant and amounted to 20.00 and 64.00 mol%, respectively. The oxides weighed in suitable proportions were homogenized and calcinated at 873, 923, 973, 1023, and 1073 K in 24 h stages. After each sintering stage the samples were powdered using agate mortar and examined with the aid of XRD. The samples obtained after last heating stage were additionally examined by the IR and DTA/TG methods. Results of investigations by XRD, DTA, and IR methods allow a determination of phase composition of samples, establishing of their melting temperatures as well as melting behavior [17–20].
Two additional samples were prepared for IR investigations. ZnV2O6 was synthesized from oxides by sintering in 24 h stages at 823, 873, and 923 K, whereas CoMoO4 from CoCO3 and MoO3 by calcination at 873, 923, and 973 K.
The DTA/TG examinations were made using an apparatus of Paulik–Paulik–Erdey type (MOM, Hungary). Samples of 500 mg were investigated in air up to the 1,273 K in quartz crucibles and at the heating rate of 10 K min−1.
X-ray diffraction phase analysis of the samples was performed using a DRON-3 diffractometer (Bourevestnik, Sankt Petersburg, Russia) applying the CoKα/Fe radiation (step 0.02° 2θ, time 1 s).
The IR spectra were recorded on a SPECORD M-80 spectrometer (Carl-Zeiss Jena, Germany) in the wave-number region of 1,500–200 cm−1. The samples were mixed with KBr in a wieght ratio of 1:300 and then pressed to pellets.
Results and discussion
The XRD experimental results have shown that diffraction patterns of all materials after the last calcination stage at 1,073 K, were similar to one another and to the diffractogram of Fe8V10W16O85 both with respect to the number and to the mutual intensity relations of the recorded diffraction lines. The angular positions of these lines were shifted with increasing the Al2O3 content in initial oxide mixtures toward higher angles 2θ, in comparison with the diffractogram of Fe8V10W16O85, i.e., they corresponded to smaller interplanar distances d. The same tendency was observed in the case of formation of solid solution of the Fe8V10W16−x Mo x O85 type (for x ≤ 4) when the Mo6+ ions were substituted for W6+ ones [14]. The obtained results indicated that continuous substitutional solid solution of a general formula Fe8−x Al x V10W16O85 is formed by an incorporation of the Al3+ ions into the crystal lattice of Fe8V10W16O85 instead of Fe3+. The formulae of obtained solid solution were evaluated from the content of initial oxides. Diffraction lines of the Al8V10W16O85 and Fe8−x Al x V10W16O85 type solid solution samples (x = 2, 4, 6) recorded within 2θ (CoKα-aver) 4–65° region were selected for indexing (program Refinement). The result of indexing of powder diffraction pattern of Al8V10W16O85 is presented in Table 1. The parameters and volumes of the unit cells of Al8V10W16O85 as well as Fe8V10W12Mo4O85 [14] and Fe8−x Al x V10W16O85 solid solutions are presented in Table 2.
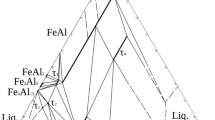
Looking for isostructural compounds with Fe8V10W16O85, Al8V10W16O85, Fe8−x Al x V10W16O85, and Fe8V10W16−x Mo x O85 solid solution phases it have been taken under consideration that their general formulas can be given as M34O85 or 17 × M2O5 (M stands for metal ion) and that these compounds have very characteristic values of unit cell parameters. The literature scan has shown that all these phases are isostructural with polymorphic form of niobium(V) oxide designated as M–Nb2O5 [21] and (W0.35V0.65)2O5, (M2O5) [22]. The values of their unit cell parameters (Table 2), the same type of general formula, M2O5, as well as very similar powder diffraction patterns support this assumption. Figure 1 presents the crystal structure of M–Nb2O5 [21]. M–Nb2O5 and (W0.35V0.65)2O5 belong to a block structure phases with ReO3 type blocks of 4 × 4×∞ dimensions. These phases are built up of corner and edge shared highly distorted MO6 octahedra. An analysis of the data compiled in Table 2 indicates that with increasing the incorporation extent of the smaller Al3+ ions into the Fe8V10W16O85 structure, the unit cell parameters a = b are decreasing whereas parameter c being almost constant.
The crystal structure of M–Nb2O5 [21]
The single phase materials obtained after the last heating stage were subjected to the DTA/TG investigation. In the DTA curve of Fe8V10W16O85 two endothermic effects were recorded up to 1,273 K with their onsets at 1,113 and 1,213 K. The second effect was much smaller than the first one and it was registered as a poorly pronounced remnant effect. It was in accord with literature data where the first endothermic effect was attributed to incongruent melting of Fe8V10W16O85 [10].
On the other hand in the DTA curves of the Fe8−x Al x V10W16O85 solid solution materials (x > 0) and Al8V10W16O85 phase one endothermic effect was recorded up to 1,273 K. Figure 2 shows the DTA curve of Al8V10W16O85. The onset temperature of the endothermic effects was increasing gradually with the increase of aluminum content. Values of temperatures were 1163, 1168, 1173, and 1183 K for x = 2, 4, 6, and 8, respectively. No weight changes were recorded on the TG curves (not presented) up to the onsets of the observed endothermic effects on the DTA curves.
In order to explain the nature of the endothermic effect on the DTA curve of Al8V10W16O85 and to establish its melting behavior the sample of this phase was additionally heated for 3 h at 1,213 K, i.e., at temperature close to the extremum temperature of the endothermic effect registered in the DTA curve. After heating at 1,213 K sample was cooled rapidly to room temperature. The X-ray phase analysis of the partially melted at this temperature sample (Fig. 3, graph b) showed that it comprised a mixture of WO3, Al2(WO4)3, and V2O5. Diffraction lines characteristic for V2O5 were shifted toward higher 2θ angles indicating formation of V2−x M x O5 (M stands for metal ion) solid solution. At 1,213 K V2O5 do not exist already as solid phase, so it crystallizes from the liquid. Thus, it was concluded that Al8V10W16O85 melts incongruently and the solid products of its melting are WO3 and Al2(WO4)3:
Fe8V10W16O85, Al8V10W16O85 as well as the solid solution phases Fe8V10W16−x Mo x O85 and Fe8−x Al x V10W16O85 were also subjected to an investigation with the use of infra-red spectroscopy (IR). The results of literature survey [21, 22] have revealed that Fe8V10W16O85 type phases are isostructural with M–Nb2O5 and (W0.35V0.65)2O5, built up from highly distorted MO6 octahedra. Since the VO6 octahedra are relatively less common polyhedra, for comparison, IR investigations have been conducted also in the case of ZnV2O6. The crystal structure of this phase is built up from distorted VO6 octahedra [23]. On the other hand, IR spectrum of CoMoO4 have been recorded because this phase has crystal structure related to these ones of M–Nb2O5 and (W0.35V0.65)2O5 [24].
Figure 4 shows the IR spectra of CoMoO4 (curve a), Al8V10W16O85 (curve b), Fe4Al4V10W16O85 (curve c), Fe8V10W16O85 (curve d) [12], Fe8V10W12Mo4O85 (curve e) [14], and ZnV2O6 (curve f).
The IR spectra of all Fe8V10W16O85 type phases are very similar, what supports assumption that these phases are isostructural.
The IR spectrum of the Fe8V10W16O85 (curve d) reveals the presence of absorption bands with their maxima at 922, 654, 486, 368, and 320 cm−1 [12–14]. With increasing the incorporation extent of the lighter and smaller Al3+ ions into the structure of Fe8V10W16O85 a relatively small shift of the respective absorption bands towardhigher wave-numbers was observed. Their maxima characteristic for Al8V10W16O85 are observed at 944, 660, 514, 412, and 334 cm−1 (curve b). A broad absorption bands lying at the range of wave-numbers 1,050–800 cm−1 are characteristic for all presented spectra (curves a–f) of phases built up only from distorted octahedra. It is noteworthy that broad absorption band in the IR spectrum of WO3 covering the wave-number region of 1,000–700 cm−1 is caused by stretching vibrations of W–O bonds in highly distorted WO6 octahedra [13, 14]. Thus, the absorption band covering the wave-number region of 1,050–800 cm−1 in the IR spectrum of the Fe8V10W16O85 type phases can be attributed to stretching vibrations of the very short M–O bonds in the MO6 (M = W, V, Al, Fe) octahedra [19]. Another very broad absorption band covering the wave-number region of 800–550 cm−1 can correspond to stretching vibrations of longer M–O bonds in MO6 octahedra. A similar absorption band with a 640 cm−1 maximum was noticed in an IR spectra of α- and γ-Fe2WO6, comprising WO6 and FeO6 octahedra in their structures [13, 14, 25]. Next absorption band recorded in the range of 550–400 cm−1 can be the most likely ascribed to stretching vibrations of Fe–O and Al–O bonds in MO6 octahedra. A similar absorption bands were noticed in the IR spectra of γ-Fe2WO6, FeVO4, and AlVO4 in the structure of which the FeO6 or AlO6 octahedra occur [7, 13, 14, 25]. On the other hand, bands lying within the wave-number range of 400–250 cm−1 may correspond to bending vibrations of M–O bonds in MO6 octahedra or be of a mixed character [14, 19]. An evident broadening of the absorption bands in the IR spectrum of Fe8V10W12Mo4O85 (curve e) in comparison to the IR spectrum of Fe8V10W16O85 is undoubtedly due to an appearance of numerous additional Mo–O bonds in the structure of Fe8V10W12Mo4O85.
Thus, analysis of the IR spectra of the Fe8V10W16O85 type phases seems to support assumption based on structural considerations that these phases are built up from MO6 octahedra.
Conclusions
In this study, the results concerning the quaternary system Fe2O3–Al2O3–V2O5–WO3 were presented for the first time. The XRD, DTA, and IR measuring techniques were used to show that:
-
1.
In the ternary system Al2O3–V2O5–WO3 new compound Al8V10W16O85 forms, not described earlier in literature.
-
2.
The Al8V10W16O85 melts incongruently at 1,183 K with deposition of Al2(WO4)3 and WO3 as a solid products of melting.
-
3.
In the quaternary system Fe2O3–Al2O3–V2O5–WO3 continuous solid solution Fe8−x Al x V10W16O85 forms, not described earlier in the literature.
-
4.
Al8V10W16O85 and Fe8V10W16O85 as well as solid solutions Fe8−x Al x V10W16O85 and Fe8V10W16−x Mo x O85 are isostructural with M–Nb2O5 and (W0.35V0.65)2O5 and belong to block type structure phases.
-
5.
With the increase of Al3+ content in the crystal lattice of Fe8V10W16O85 a decrease of the a = b parameters of the unite cell occurs, with c parameter being almost constant.
-
6.
The melting temperature of the Fe8−x Al x V10W16O85 solid solution increase with increasing the content of Al2O3 and changes from being equal to 1,113 K for x = 0 up to 1,183 K for x = 8.
-
7.
Analysis of the IR spectra of the Fe8V10W16−x Mo x O85 type phases supports assumption that these phases are built up from VO6, WO6, FeO6, and AlO6 octahedra.
References
Eckert H, Wachs IE. Solid state 51V NMR structural studies on supported vanadium(V) oxide catalysts: vanadium oxide surface layers on alumina and titania supports. J Phys Chem. 1989;93:6796–805.
Vuurman MA, Stufkens DJ, Oskam A, Deo G, Wachs IE. Combined Raman and IR study of MO x –V2O5/Al2O5 (MO x = MoO3, WO3, NiO, CoO) catalysts under dehydrated conditions. J Chem Soc Faraday Trans. 1996;92(17):3259–65.
Guerrero-Perez MO, Herrera MC, Malpartida I, Larrubia MA, Alemany LJ. Characterization and FT-IR study of nanostructured alumina-supported V–Mo–W–O catalysts. Catal Today. 2006;118:360–5.
Mitra B, Wachs IE, Deo G. Promotion of the propane ODH reaction over supported V2O5/Al2O3 catalyst with secondary surface metal oxide additives. J Catal. 2006;240:151–9.
Craig DC, Stephenson NC. A structural study in the system Al2O3–WO3. Acta Cryst. 1968;B24:1250–5.
Hanuza J, Maczka M, Hermanowicz K, Andruszkiewicz M, Pietraszko A, Strek W, Deren P. The structure and spectroscopic properties of Al2−x Cr x (WO4)3 crystals in orthorhombic and monoclinic phases. J Solid State Chem. 1993;105:49–69.
Dabrowska G, Tabero P, Kurzawa MJ. Phase relations in the Al2O3–V2O5–MoO3 system in the solid state. The crystal structure of AlVO4. J Phase Equilib Differ. 2009;30(3):220–9.
Tarama K, Teranishi S, Yoshida S. Study on the reduction process of vanadium oxide catalysts by means of infrared spectroscopy and X-ray diffraction. Bull Inst Chem Kyoto Univ. 1968;5:185–97.
Darriet J, Galy J, Hagenmuller P. Mixed oxides of bronze structure M x V2−y T y O5 (T = Mo, W). I Li x V2−y T y O5. J Solid State Chem. 1971;3:596–603. (in French).
Walczak J, Rychlowska-Himmel I. Investigation of the real composition of the phase formed in the Fe2O3–V2O5–WO3 system. J Mater Sci. 1994;29:2745–50.
Rychlowska-Himmel I. Phase equilibria in the system Fe2O3–V2O5–WO3 in the solid state. J Therm Anal Calorim. 2000;60:173–6.
Walczak J, Rychlowska-Himmel I, Tabero P. Reaction mechanism of Fe8V10W16O85 synthesis. J Therm Anal Calorim. 1999;56:419–22.
Rychlowska-Himmel I, Tabero P. Phase equilibria in the system V2O5–Fe8V10W16O85 and some properties of the Fe8V10W16O85 phase. J Therm Anal Calorim. 2001;65:537–43.
Tabero P. Formation and properties of the Fe8V10W16−x Mo x O85 type solid solution. J Therm Anal Calorim. 2007;88:37–41.
Walczak J, Rychlowska-Himmel I. Phase equilibria in the Fe8V10W16O85–Fe2O3 and Fe8V10W16O85–Fe2WO6 systems. J Therm Anal Calorim. 1998;54:867–72.
Walczak J, Rychlowska-Himmel I. Phase diagram of the FeVO4–Fe2WO6 system. Thermochim Acta. 1994;239:269–74.
Blonska-Tabero A. New phase in the system FeVO4–Cd4V2O9. J Therm Anal Calorim. 2008;93:707–10.
Bosacka M, Blonska-Tabero A. Reinvestigation of system CdO–V2O5 in the solid state. J Therm Anal Calorim. 2008;93:811–5.
Tomaszewicz E, Typek J, Kaczmarek SM. Synthesis, characterization and thermal behaviour of new copper and rare-earth metal tungstates. J Therm Anal Calorim. 2009;98:409–21.
Filipek E, Wieczorek-Ciurowa K. Comparison between the synthesis in molybdenum and antimony oxides system by high-temperature treatment and high-energy ball milling. J Therm Anal Calorim. 2009;97:105–10.
Mertin W, Andersson S, Gruehn R. The crystal structure of M–Nb2O5. J Solid State Chem. 1970;1:419–24. (in German).
Israelsson M, Kihlborg L. (W0.35V0.65)2O5, a new, simple block structure. Ark Kemi. 1968;30(12):129–40.
Andretti GD, Calestani G, Montenero A. Refinement of the crystal structure of ZnV2O6. Z Kristallogr. 1984;168:53–8.
Smith G, Ibers JA. The crystal structure of cobalt molybdate, CoMoO4. Acta Cryst. 1965;19:269–73.
Senegas J, Galy J. Crystal structure of double oxide Fe2WO6. J Solid State Chem. 1974;10:5–11. (in French).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tabero, P. Formation and properties of the new Al8V10W16O85 and Fe8−x Al x V10W16O85 phases with the M–Nb2O5 structure. J Therm Anal Calorim 101, 561–566 (2010). https://doi.org/10.1007/s10973-010-0848-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-0848-z