Abstract
In this study, the zeolitic tuffs having clinoptilolite obtained from Bigadic region of western of Anatolia, Turkey were investigated as regards to whether it is possible to be transformed into amorphous phase from them. At first, the zeolite tuffs rich in clinoptilolite were characterized using XRD, DTA, TG, DSC, and FTIR standard methods. All the samples were heated at 110 °C for 2 h and then were expanded within 5 min between the temperatures 1200 and 1400 °C. In addition, porosity and density were determined. The resistance values of all the samples were measured in acidic and basic media. These samples were also analyzed. As a result of this study, zeolitic tuffs in clinoptilolite were transformed into amorphous phase, and especially in chemical industry were found convenient.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Natural zeolites are found in volcanic ashes as well as sediment rocks. They are associated with clay minerals, borates, carbonates, and soda minerals similar to many of those found in other parts of the world [1]. The reserve of zeolite in Turkey is about 50 billion tons. Zeolite tuffs rich in clinoptilolite are found especially in Bigadiç (Balıkesir). The most important natural zeolites which are also quite abundant are clinoptilolite, mordenite, analcite, and chabazite [2]. Clinoptilolite, one of the most common natural zeolite mineral, is a member of the heulandite group [3, 4]. Zeolites are hydrated aluminosilicates of alkali and alkaline earth elements with unique crystal structures consisting of a three-dimensional framework of SiO4 and AlO4 tetrahedral [5]. The isomorphic substitution of Si by Al leads to a negative charge density in the zeolite lattice. This charge is neutralized by introducing exchanged monovalent, divalent or trivalent cations in the structural sites of the zeolite [6]. The mobile non-framework cations are placed in cavities in the channel walls and coordinated with the water molecules within the channel. Zeolites which are also known as moleculer sieves, are crystalline solids having pores with diameters of 3–10 Å [7, 8]. Owing to their chemical, physical, and structural properties, zeolites act as a molecular sieve and also as an ionic exchanger [9, 10]. FITIR spectra of clinoptilolite are divided into two classes. The first class of vibrations arising due to internal vibrations of the TO4 tetrahedron are called asymmetry stretch O–Si(Al)–O, symmetry stretch, and T–O double ring, which are observed at 1251–950, 750–650, 500–420 cm−1 wavelengths, respectively. The second class of vibrations, namely, T–O double ring, pore opening, symmetry stretch, and asymmetry stretch related to the linkages between the tetrahedral are observed at 650–500, 420–300, 750–820, and 1150–1050 cm−1 wavelengths, respectively. In addition, H-bonded H2O, H–O, and isolated OH stretching are at 3400 and 3700 cm−1 wavelengths, respectively [11–13]. Ion exchange process causes formation of new bond and structure of zeolites which can change due which could be detected by FITIR [13, 14].
Clinoptilolite contains three channels, limited by a system of tetrahedral rings: two channels of eight and ten tetrahedral parallel to c axis of the structure, and a third channel formed by eight-member rings and connected to the other two channels. The large central cavities and entry channels of zeolites are filled with water molecules forming hydration spheres around the exchangeable cation. In the zeolite framework, each aluminum atom introduces one negative charge on the framework which must be balanced by an exchangeable cations (Ca2+, Ma2+, Na+, K+, etc.). The exchangeable cations located within the framework play a crucial role in adsorption and thermal properties of the zeolites [4]. Since water is selectively adsorbed by natural zeolite, it hinders the adsorption of the other molecules [4]. The water may be removed usually by heating to 350 or 400 °C for a few hours or overnight [15]. Zeolites’ unique market position is sustained by the continuous development of their ion exchange and adsorption properties, especially through their surface treatment [16–18]. Natural zeolites are used for making lightweight aggregates. The high water content of zeolites makes them as very interesting materials for production of lightweight aggregates [19]. This feature sensibly reduces the bulk density and makes it easier to work with and transport this product. In this study, natural zeolitic tuffs were used to be transformed into amorphous phase.
Experimental
The zeolitic tuffs rich in clinoptilolite were obtained from Bigadiç reserves (Balıkesir) region, Turkey. They were crushed into small pieces from rock forms and then powdered in a mortar. The zeolitic tuff samples of smaller than 125 mm sizes (14 meshes, ASTM 11–70) were calcinated at 110 °C for 2 h. These are observed to expand within 5 min in the temperature range of 1200–1400 °C. The mineralogical composition of materials mainly consists of clinoptilolite. The associated minerals for natural Bigadic zeolitic tuff rich in clinoptilolite is quartz [20]. The chemical composition of zeolitic tuffs rich in clinoptilolite is given in Table 1. The quantitative clinoptilolite content of the sample is 90% in sample mass. In addition, determination of pH, porosity, hardness, and density of the samples was accomplished. XRD analyses of the samples were performed using Rigaku RINT-2200 diffractometer in a scanning range of 5–40o (2θ) at a rate of 2o (2θ) min−1. Cu Kα radiation was used. TG–DTA curves were measured from 30 to 1000 °C at a rate of 10 °C min−1 with Setsys Evolution Setaram thermal analysis apparatus. DSC was carried out on a Setaram DSC-151R analyzer. Sample powder was scanned with a heating rate of 5 °C min−1 up to 550 °C. The scanning electron photograph (SEM) was obtained on a ZEISS EVO 50 EP electron microscope. The FTIR spectra in the 4000–500 cm−1 range were recorded at room temperature using Bruker IFS 66 V/S spectrometer equipped with Opus software. Spectra were collected at 4 cm−1 resolution. Samples were prepared by the standard KBr pellet methods. The KBr was dried at 200 °C for 24 h, and then 100 mg KBr was homogenized with 1 mg zeolite samples.
Results and discussion
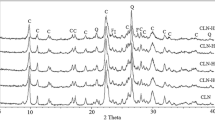
Comparative experimental results of natural and expanded zeolitic tuffs are given in Table 2. The density of expanded samples is found to expand approximately three times compared with that of natural zeolite. The pH, porosity, hardness, and density range values of the natural zeolitic tuff were found to be 8.5, 0.34, 3 on the Mohs scale, and 1.7–2.1 × 103 kg m−3 respectively. The density of expanded samples obtained was between 0.5 and 0.98 × 103 kg m−3. The resistance tests of expanded samples were done in acidic and basic media. It is noticeable from Table 2 that the density of expanded zeolitic tuff is lower, and it is highly resistant in acidic and basic media. The hardness of the expanded natural zeolitic tuff is determined to be about 6 on the Mohs scale. The X-ray diffraction diagram depicted in Fig. 1a exhibits characteristic clinoptilolite peaks at 2θ = 9.87, 22.4 and 30o [21]. As seen from Fig. 1b, the surface of expanded zeolite was transformed to amorphous phase, and it was also evidenced by XRD analysis. The water concentrations in the samples were determined from the TG curve mass loss (Fig. 2). The DTA curve of natural Bigadic zeolite sample displays a single endothermic peak at 128 °C as a result of a single step dehydration process. The total mass loss for sample determined by TG analysis is 11.72%. Most of the physisorbed water was lost between 30 and 200 °C, and in the broad interval between 200 and 500 °C, more strongly associated water was lost. The DSC curve of natural zeolitic tuff shows two endotherms at 91 and 441 °C (Fig. 3). The FTIR spectra were recorded after dehydration of natural zeolitic tuff, at various fixed temperatures for 6 h (110, 350, and 900 °C) (Fig. 4). The band at 1050–1200 cm−1 region as shown in Fig. 4 is assigned to the T–O stretching vibration. Moreover, the peaks that appeared around 1100–1200 cm−1 were due to the OH− bending bands. That the natural zeolitic tuff contained H2O molecules in its structure could not be determined even when it was heated up to 900 °C. SEM micrograph of the expanded zeolitic tuff is presented in Fig. 5.
Conclusions
The occurrence in Turkey of huge zeolite deposits motivated this study to appraise the behaviour of zeolitic tuff as raw materials. In this study, natural zeolitic tuffs were used for being transformed to amorphous phase. The density of the expanded zeolitic tuff was determined to be in the range of 0.5–0.98 × 103 kg m−3. The density of expanded zeolitic tuff is found to be lower than that of natural zeolitic tuff, and it is also found that it is highly resistant in acidic and basic media. The usage of expanded zeolitic tuffs is advantageous, especially in chemical industry, by virtue of its convenience. In addition, the expanded zeolitic tuff can be used in the production of lightweight materials, glass etc. without any gas releasing materials.
References
Birsoy R. Activity diagrams of zeolites: implications for the occurrences of zeolites in Turkey and erionite worldwide. Clay Clay Miner. 2002;50:136–44.
Mumpton FA. Natural zeolites: a new mineral commodity. In: Sand LB, Mumpton FA, editors. Natural zeolites: occurrence, properties, use. Elmsford: Pergamon Press; 1978. p. 3–27.
Breck DW. Zeolites: molecular sieves. New York: Wiley-Interscience; 1980.
Cakıcıoglu-Ozkan F, Ulkü S. Diffusion mechanism of water vapour in a zeolitic tuff rich in clinoptilolite. J Therm Anal Calorim. 2008;94:699–702.
Dyer A. An introduction to zeolite molecular sieves. New York: Wiley; 1988.
Breck DW. Zeolite molecular sieves. New York: Wiley; 1984.
Breck DW. Zeolites molecular sieves: structure, chemistry, and use. New York: Wiley; 1974.
Erdogan B, Sakızcı M, Yörükoğulları E. Investigation of clinoptilolite rich natural zeolites from Turkey: a combined XRF, TG/DTG, DTA and DSC study. J Therm Anal Calorim. 2009. doi:10.1007/s10973-009-0118-0.
Barrer RM. Zeolites and clay minerals as sorbents and molecular sieves. London: Academic Press; 1978.
Erdogan B, Sakızcı M, Yörükoğulları E. Characterization and ethylene adsorption of natural and modified clinoptilolites. Appl Surf Sci. 2008;254:2450–7.
Conception-Rosabal B, Rodrigues-Fuentes G, Bogdanchikova N, Bosch P, Avalos M, Lara VH. Comparative microbiological activity of silver modified natural clinoptilolites. Microporous Mesoporous Mater. 2005;86:249–55.
Afzal M, Yasmeen G, Saleem M, Butt PK, Khattak AK, Afzalı J. TG and DTA study of the thermal dehydration of metal-exchanged zeolite-4A samples. J Therm Anal Calorim. 2000;62:277–84.
Akdeniz Y, Ülkü S. Thermal stability of Ag-exchanged clinoptilolite rich mineral. J Therm Anal Calorim. 2008;94:703–10.
Costaldi P, Santona L, Coza C, Giuliano V, Abbruzzese C, Nastro V, et al. J Mol Struct. 2005;734:424.
Yörükoğulları E, Yılmaz G, Sakızcı M, Erdoğan B. The usability of natural zeolites for lightweight aggregate production. In: Heinrich JG, Aneziris C, editors. Proceedings of the 10th ECERS Conference. Baden-Baden: Göller Verlag; 2007. p. 1276–8.
Crini G, Morcellet M. Synthesis and applications of adsorbents containing cyclodextrins. J Sep Sci. 2002;25:789–813.
Zeocem, a.s., 0904 34 Bystré 282, Slovakia. www.zeocom.sk.
Chmielewska E, Sabova L, Jesenak K. Study of adsorption phenomena ongoing onto clinoptilolite with the immobilized interfaces. J Therm Anal Calorim. 2008;92:567–71.
Torii K. Utilization of natural zeolites in Japan, in natural zeolites, occurrence, properties, use. In: Sand LB, Mumpton FA, editors. Natural zeolites: occurrence, properties, use. Elmsford: Pergamon Press; 1978, p. 441–50.
Dikmen S. Adsorption of natural gas (methane) on natural zeolites. MS thesis, Institute of Pure and Applied Science, Anadolu University, Turkey; 1998.
Gottordi G, Galli E. Natural zeolites: mineral and rock. Berlin: Springer Verlag; 1985. p. 266–7.
Acknowledgements
The authors thank Dr. M. Sakızcı and B. Erdoğan (Anadolu University Department of Physics, Eskişehir, Turkey) for the measurement of the XRD, DTA, TG, DSC and FTIR standard methods.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yörükoğulları, E., Yılmaz, G. & Dikmen, S. Thermal treatment of zeolitic tuff. J Therm Anal Calorim 100, 925–928 (2010). https://doi.org/10.1007/s10973-009-0503-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0503-8