Abstract
This study presents the results of the methane adsorption properties of clinoptilolite tuff from Bigadic, Turkey and that of acid treated forms at 273 and 293 K up to 100 kPa using volumetric apparatus. In order to assess changes in structural and gas adsorption properties of clinoptilolite, zeolite sample was treated with acid solutions of varying concentrations (0.1, 0.5, 1.0 and 2.0 M) at 70 °C during 3 h. Structural and thermal characterization of natural and acid treated clinoptilolite samples were carried out using a combination of techniques such as X-ray diffraction, X-ray fluorescence, thermogravimetric, differential thermal analysis and nitrogen adsorption methods. At both temperatures, uptake of methane (CH4) increased in the following order: CLN < CLN-H2 < CLN-H1 < CLN-H05 < CLN-H01. CH4 adsorption capacities of the original and acid treated clinoptilolites were found in the range of 0.476–0.910 mmol/g and 0.398–0.691 mmol/g at 273 and 293 K, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Zeolites are porous, crystalline, hydrated aluminosilicates with the framework structure consisting of AlO4 and SiO4 tetrahedra (Breck 1984). These are linked to each other by sharing all of the oxygens to form the zeolite structure containing channels. The net positive charge deficiency arised from substitution of an AlO4 tetrahedron for a SiO4 tetrahedron is balanced by exchangeable cations within these channels (Breck 1984; Dyer 1988). The size, number and distribution of the exchangeable cations affect the adsorption properties of the zeolite minerals (Barrer 1978). Clinoptilolite, a member of heulandite group, is a crystalline, naturally occurring zeolite mineral (Gottardi and Galli 1985). Clinoptilolite consists of a two dimensional system of three types of channels with sizes of 4.4 × 7.2, 4.0 × 5.5 and 4.1 × 4.0 Å, respectively (Koyama and Takeuchi 1977; Galli et al. 1983). Channels A (10-member rings) and B (8-member rings) are parallel to each other and to the c-axis of the unit cell, while C channels (8-member rings) lie along the a-axis intersecting both A and B channels (Merkle and Slaughter 1968; Gottardi and Galli 1985). These channels are occupied by charge balancing cations and water molecules (Merkle and Slaughter 1968; Ackley et al. 1992). Clinoptilolites are extensively used in a wide variety of adsorption and environmental applications because of their availability, relatively low cost and high chemical stability.
One of the most common applications of clinoptilolites is their acid treatment with the mineral acid solutions such as HCl and H2SO4. Acid treatment causes the exchange of H+ ions with extra framework cations of clinoptilolite and dealumination (hydrolysis of Al–O–Si bonds) (Rozic et al. 2005; Garcia-Basabe et al. 2010). Acid treatment also leads to a modification in zeolite morphology, by the destruction of channel-blocking impurities and the development of the secondary porosity (Rozic et al. 2005). As the concentration of the acid solution increases, Si/Al ratio, surface area and the number of strong acid sites increase (Christidis et al. 2003; Radosavljević-Mihajlović et al. 2004). Because the adsorption properties of acid treated zeolites mainly depend on the concentration and nature of acids and on the contact time of acid solutions with the zeolite, acid treatment conditions must be controlled in order not to cause the partial and total destruction of the zeolite framework.
Methane is a colorless, odorless and flammable gas with the chemical formula CH4 (Cardarelli 2008). It is the simplest alkane and occurs in nature as a major component of natural gas, as a constituent of firedamp in coal mines. Methane also called marsh gas since it is formed by anaerobic bacterial decomposition of vegetable matter under water (Patnaik 2010). In the enclosed space, methane in high concentrations may displace the oxygen for breathing and reduced oxygen intake can cause headache, nausea, suffocation, loss of consciousness and ultimately, brain death (Carson 2002). Methane is also potentially dangerous as an explosive compound and a greenhouse gas that contributes to potential global warming (Shindell et al. 2009). It has a long lifespan in the atmosphere; therefore methane also contributes the depletion of the ozone layer (Hamzah et al. 2010).
The relative abundance of methane makes it an attractive fuel, although storage of it reveals challenges owing to its gaseous state found at normal conditions. The adsorption of methane on synthetic zeolites such as 13X, 4A, 5A, G5, NaX, NaY (Grande and Blom 2014; Ahmed and Theydan 2014; Ding et al. 1988; Cavenati et al. 2004; Triebe et al. 1996, Berlier et al. 1995; Choudhary et al. 1995), natural zeolites such as erionite, clinoptilolite and mordenite (Aguilar-Armenta et al. 2001, 2003; Predescu et al. 1995; Hernández-Huesca et al. 1999; Jayaraman et al. 2005; Macedonia et al. 2000; Ackley and Yang 1991; Aguilar-Armenta and Romero-Perez 2009; Maple and Williams 2008) and zeolite-like materials (Antoniou et al. 2014) has been investigated worldwide. Based on the studies in the literature, it is concluded that the studies on the methane adsorption by acid treated natural zeolites is especially rare. Large deposits of clinoptilolite tuff are widespread in Turkey, whilst there are limited studies on methane adsorption properties of acid modified forms of clinoptilolite from Turkey. Hence, the purpose of the present study was to investigate the influence of the HCl treatment on the thermal, structural and methane adsorption properties of natural clinoptilolite and to evaluate their capacities in the removal of methane at 273 and 293 K for possible applications in methane storage.
2 Experimental
2.1 Materials and methods
Clinoptilolite-rich mineral (CLN) used in this study originated from Bigadic, Turkey. First, clinoptilolite sample was crushed and sieved to obtain <63 μm fractions. In order to assess the influence of acid treatment on clinoptilolite tuff for CH4 removal, samples were treated with HCl acid in the following way. Clinoptilolite samples were modified by batch method using 100 ml of HCl solutions (0.1, 0.5, 1.0 and 2.0 M) at 70 °C for 3 h. After the acid treatment, the samples were separated and washed with hot distilled water several times until the filtrate was free from chloride ions and then dried at room temperature. Before the experimental procedure, all samples were dried in an oven at 150 °C for 24 h and stored in a desiccator. The resulting acid-treated samples were named as CLN-H01, CLN-H05, CLN-H1 and CLN-H2, according to their corresponding 0.1, 0.5, 1.0 and 2.0 M HCl treatments. HCl was supplied by Merck (Darmstadt, Germany) and all solutions were prepared using de-ionized water.
2.2 Instrumentation
Prior to the study of methane adsorption, the structural and thermal properties of clinoptilolite-rich mineral and that of acid modified forms were determined by using XRD, XRF, TG-DTG, DTA and N2 adsorption techniques. Identification of mineral components in the clinoptilolite tuff was performed using XRD technique and the XRD diffractograms were obtained with a Rigaku, RINT-2200 model instrument, using CuKα radiation (λ = 1.54 Å), 40 kV and 30 mA power supply. XRD patterns were collected from 5o to 40o. Chemical compositions were evaluated on powdered samples fused with lithium tetraborate using XRF analysis (Rigaku ZSX Primus instrument). The specific BET surface areas were calculated from the first part of the N2 adsorption isotherm (0.05 < P/P0 < 0.30) obtained at 77 K with N2 in Autosorb-1C equipment (Quantachrome Instruments, U.S.A.) previously degassed at 300 °C for 7 h prior to measurement. The micropore area and volume were calculated by the t-plot method. The cumulative pore volume and average pore diameter were calculated using the adsorption data by the DFT (Density Functional Theory) model. High-purity (99.99 %) nitrogen was used in adsorption measurements. TG, DTG and DTA curves were obtained simultaneously with a Setsys Evolution Setaram thermal analyzer at a heating rate of 10 °C/min in the temperature range 30–1000 °C. Approximately 40 mg of sample was used in each run. The adsorption isotherms of CH4 on natural and acid modified clinoptilolite-rich minerals were determined using automated Autosorb 1 volumetric equipment at 273 and 293 K up to 100 kPa. The methane gas used was highly pure (purity higher than 99 %). Before the study of methane adsorptions, samples were degassed in vacuum at 300 °C for 7 h. All the experimental runs were repeated in order to confirm desired reproducibility of the obtained results.
3 Results and discussion
3.1 Element composition
The chemical compositions of the CLN and that of acid-treated (CLN-H01, CLN-H05, CLN-H1 and CLN-H2) forms, expressed in terms of oxide species, are given as % mass in Table 1. The presence of elements having smaller concentrations than Al and Si was observed. The CLN was characterized by high potassium and calcium and low magnesium and sodium contents. In addition, iron is present associated with impurities present in zeolitic tuffs such as iron oxides. As seen from the XRF data, both the significant amount of exchangeable cations (Na+, K+, Ca2+, Mg2+) and framework aluminum were progressively removed from the clinoptilolite tuff as the concentration of HCl increased. Similar results were found by other authors (Radosavljević-Mihajlović et al. 2004; Çakıcıoğlu-Özkan and Ülkü 2005). Atom numbers per unit cell and Si/Al ratios of natural and that of acid treated forms, assuming 72 oxygens, are presented in Table 2. Si/Al ratio of natural clinoptilolite is 5.0, which is in good agreement with values presented by other workers (Petrakakis et al. 2007; Hernandez-Beltran and Olguin 2007). Si/Al ratio of CLN increased from 5.0 to 7.5 in the CLN-H2 sample treated with 2.0 M HCl solution. XRF data confirmed that the treatment with HCl acid produced significant modifications in the composition of clinoptilolite tuff.
3.2 X-ray analysis
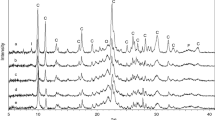
In order to indicate the influence of HCl treatment on the crystal structure of clinoptilolite, the XRD patterns of all the samples (CLN, CLN-H01, CLN-H05, CLN-H1 and CLN-H2) are shown in Fig. 1. CLN sample exhibited high crystallinity, with the characteristic reflection peaks attributed to the clinoptilolite as a main mineral component at 2θ = 9.88o, 22.47o and 30.06o (Moore and Reynolds 1997) and denoted by “C” on the XRD patterns. In addition to the clinoptilolite phase (80–85 %), the presence of quartz (12–14 %) and minor quantities of feldspar (3–4 %) and mica-illite (1–2 %) were also determined using the method given by Esenli and Sirkecioğlu (2005).
As seen from the XRD data, acid treatment using concentrations up to 0.5 M have no considerable effect on the crystallinity, whilst samples treated with higher acid concentrations (1.0 and 2.0 M), showed a decrease in the intensity of the main clinoptilolite peaks, indicating a loss of crystallinity of the zeolitic tuff. After the acid treatment with 2 M HCl solution (CLN-H2), the main clinoptilolite peaks still present but those of intensities were very weak (in comparison with quartz and feldspar). The broad baseline (especially for CLN-H2 sample) is indicative of the transformation of the zeolite into an amorphous phase. In addition, some slight changes in the position of the peaks were observed in the XRD patterns of acid treated samples. This can be related to both dealumination and the partial breakdown of the structure of the clinoptilolite. Similar changes after acid treatment of clinoptilolite were also observed by other authors (Garcia-Basabe et al. 2010; Radosavljević-Mihajlović et al. 2004; Arcoya et al. 1994; Salvestrini et al. 2010). As seen from Fig. 1, the insoluble impurities such as quartz and feldspar remained in the samples with the increase of the acid concentration.
3.3 Specific surface area
Nitrogen adsorption isotherms of the natural and acid treated clinoptilolite samples are shown in Fig. 2 (relative pressure P/Po vs. adsorbed volume in cc per gram of zeolite). The N2 adsorption isotherms are of the type II (Brunauer et al. 1940; Gregg and Sing 1982). BET surface areas were calculated from N2 adsorption isotherms (0.05 < P/Po < 0.30) obtained at 77 K with high-purity (99.99 %) nitrogen. Data in Fig. 2 shows that the N2 adsorption capacities of the CLN-H01, CLN-H05, CLN-H1 and CLN-H2 samples are higher than the CLN sample. The influence of acid treatment on the specific surface area, the micropore volume, the micropore area, the cumulative pore volume and the average pore diameter values were investigated and the results were reported in Table 3. The relatively low values of specific surface area (19.92 m2/g) of CLN compared to the acid treated samples are due to the fact that N2 cannot enter into the pores of the natural zeolite (about 4 Å) and it is mainly adsorbed on the external surface of the material due to the restricted diffusion caused by exchangeable cations in the channels of clinoptilolite (Tsitsishvili 1973; Ackley and Yang 1991; Ackley et al. 1992).
In the case of 2.0 M HCl treatment, the specific surface area of CLN increased from 19.92 to 117.60 m2/g and average pore diameter of CLN decreased from 189.21 to 53.51 Å (Table 3). The considerable increase in the specific surface area, micropore volume, micropore area of CLN-H2 can be attributed to opening of the windows formed by the substitution of exchangeable cations by H+, dealumination and the partial breakdown of the structure of the clinoptilolite [as shown by XRF (Table 1) and XRD analysis (Fig. 1), respectively]. Therefore, nitrogen was able to diffuse more easily and to be adsorbed more densely in acid treated clinoptilolite cavities than in original form of clinoptilolite. The observed decrease in the average pore diameter after acid treatment can be attributed to formation of secondary micropores by dissolution of free linkages. These results are in agreement with previously reported studies for clinoptilolites treated with acid solutions (Petrakakis et al. 2007; Arcoya et al. 1994).
3.4 Thermal properties
Figure 3 shows TG, DTG and DTA curves of the CLN and that of acid modified (CLN-H01, CLN-H05, CLN-H1 and CLN-H2) forms. The DTA curves for all the clinoptilolite samples were essentially similar and displayed a single endotherm at temperature ranging from 144 to 157 °C as a result of a single-step dehydration process. These endothermic peaks related to the elimination of physically adsorbed water and loosely bonded water are observed up to 200 °C, (Salvestrini et al. 2010). Clinoptilolites belonging to the zeolite group do not show major structural changes during dehydration processes which exhibit continuous mass-loss curves as a function of temperature. Clinoptilolite structure remains stable up to temperatures 600–800 °C (Mumpton 1960). As seen from Fig. 3, TG analysis of all the zeolites exhibited that no noticeable breaks occurred in the mass-loss curves as a function of temperature and the main structural changes occur at temperatures below or around 400 °C while the mass loss was continuous during heating up to 700 °C. Mass losses (%) of the natural and acid modified Bigadic clinoptilolites used at different temperature ranges were presented in Table 4. The major and rapid mass loss up to 200 °C was associated with the loss of physically adsorbed water, the water residing in the zeolite cavities and bound to the extra-framework cations. In the broad interval between 200 and 500 °C more strongly associated water is lost. Collapse of the zeolite structure happens with further water loss at the higher temperatures. In the temperature range from 500 to 700 °C, the rest of the water is gradually removed. DTG curves of the CLN, CLN-H01, CLN-H05, CLN-H1 and CLN-H2 samples exhibited a single endothermic peak in the temperature range from 30 to 350 °C (Fig. 3).
Water content decreases in the order of CLN > CLN-H01 > CLN-H05 > CLN-H1 > CLN-H2. TG data exhibits that CLN has the greatest mass loss (9.98 %) in the temperature interval 30–1000 °C compared to those of the acid modified forms. The relative mass losses of acid modified forms decrease with the increasing concentration of HCl treatment. Zeolite dehydration strongly depends on the extra framework cations in the cavities, the loss of exchangeable cations and dealumination due to the acid treatment, for this reason water content of acid treated samples decreases. In the case of CLN-H2, the partially amorphization is likely to be the cause of the decrease in the number of water molecules present. Experimental results of thermal analyses are in agreement with literature (Garcia-Basabe et al. 2010; Korkuna et al. 2006).
3.5 Adsorption of CH4
The CH4 adsorption isotherms of CLN and acid modified clinoptilolite samples (CLN-H01, CLN-H05, CLN-H1 and CLN-H2) were obtained volumetrically at 273 K and 293 K up to 100 kPa (Figs. 4, 5). High purity CH4 gas was used as the adsorbate. Experimental isotherms are similar in shape and have classic isotherm forms. Besides the structure of the adsorbent, modification process, surface area and the size of adsorbed gas are the main factors affecting the adsorption capacity (Gregg and Sing 1982). Methane [kinetic diameter 3.8 Å (Lide 2003)] can pass through the pore openings of clinoptilolite. The absolute amounts adsorbed per gram of natural and acid treated clinoptilolites were given in Table 3. The capacities of the clinoptilolite samples towards CH4 ranged from 0.476 to 0.910 mmol/g and from 0.398 to 0.691 mmol/g at 273 and 293 K up to 100 kPa, respectively. As the temperature increases, the total amount adsorbed decreases, as expected. It was observed that uptake of CH4 on all the clinoptilolite samples at both temperatures decreased in the order: CLN-H01 > CLN-H05 > CLN-H1 > CLN-H2 > CLN.
The data listed in Table 3 showed that CLN sample exhibited much lower CH4 adsorption capacity compared to acid modified samples at both temperatures owing to diffusive restrictions in clinoptilolite channels resulting from channel blockage by exchangeable cations. Experimental results showed that the treatment of natural clinoptilolite with HCl acid solutions led to an increase of adsorption capacity for CH4. In particular, the most effective adsorption of CH4 was demonstrated by clinoptilolite treated with 0.1 M of HCl solution. Under optimum condition, CH4 adsorption capacity of CLN-H01 sample is 0.910 and 0.691 mmol/g at 273 and 293 K up to 100 kPa, respectively. This abrupt increment in adsorbed amount of CH4 can be due to the exchange of metal cations by small size H+ ions, together with the elimination of some impurities, also resulting in more available space within the zeolite. The natural Bigadic clinoptilolite in this study (0.398 mmol/g at 293 K) showed higher CH4 adsorption capacity than natural clinoptilolite from Catilla, Cuba (0.120 mmol/g at 298 K) investigated by Arcoya et al. (1996). Whilst, the retention values of CH4 by natural (0.398 mmol/g) and acid treated Bigadic clinoptilolites in this study (0.463–0.691 mmol/g) at 293 K were lower than the natural and acid modified clinoptilolite (ca. 0.8 mmol/g at 300 K) studied by Ackley and Yang (1991), a raw clinoptilolite from Greece (ca. 2.5 mmol/g at 298 K) investigated by Kouvelosa et al. (2007) and H-CLI sample (1.254 mmol/g at 298 K) studied by Arcoya et al. (1996) probably due to the structural and textural properties.
The adsorption differences between the acid forms of clinoptilolite can be attributed to various degrees of accessibility of channels for CH4 molecule. With the increasing acid concentration (above 0.5 M), adsorption capacities of clinoptilolite samples decrease. The tendency of adsorption capacity decreases with significant loss of exchangeable cations and higher extent of dealumination (treatment with 1.0 and 2.0 M acid solutions), which is probably due to the change of the role of the electrostatic and Vander Waals interactions between the zeolite surface and the CH4 molecule as well as the partial amorphization of clinoptilolite. This is consistent with the data obtained by XRD data (Fig. 1). It was observed that the adsorption capacities of the H-form of clinoptilolites depend on the concentration of the acid during modification and the resulting induced structural changes. Experimental results showed that the CLN-H01 form of clinoptilolite obtained without its considerable dealumination is the most optimal for the CH4 removal in comparison with the natural and other acid modified forms studied in this work.
4 Conclusions
This study presents the effect of acid treatment on the CH4 adsorption capacity of natural Bigadic clinoptilolite for its potential applications as a sorbent. From the combined study of the natural and acid treated clinoptilolites by XRD, XRF, TG/DTG, DTA and N2 adsorption it is concluded that:
-
Treatment of the clinoptilolite with HCl acid and the subsequent washing with de-ionized water caused the significant removal of exchangeable cations and the partial dealumination. Thus, Si/Al ratios have progressively increased with increasing acid concentrations.
-
XRD analysis showed that a significant reduction in the crystallinity of zeolite was affected only when the acid concentration was 1.0 M and above. The intensities of the clinoptilolite peaks decreased with increasing acid concentration, indicating a loss of crystallinity of the zeolitic tuff.
-
The DTA curves for all samples were essentially similar and samples displayed a single endothermic peak at temperature ranging from 144 to 157 °C related to a single-step dehydration process.
-
Acid treatment changes the structure of clinoptilolite considerably, resulting in an increase of the specific surface area, the micropore area, the micropore volume and a decrease of the average pore diameter, creating the additional micropores and amorphization of the clinoptilolite tuff.
-
Clinoptilolite treated with 0.1 M HCl appeared to be a particularly suitable material for removing CH4. Further increase of acid concentrations beyond 0.1 M did not improve the removal efficiency.
Abbreviations
- CLN:
-
Original clinoptilolite
- CLN-H01:
-
Clinoptilolite treated with 0.1 M HCl solution
- CLN-H05:
-
Clinoptilolite treated with 0.5 M HCl solution
- CLN-H1:
-
Clinoptilolite treated with 1.0 M HCl solution
- CLN-H2:
-
Clinoptilolite treated with 2.0 M HCl solution
References
Ackley, M.W., Yang, R.T.: Adsorption characteristics of high-exchange clinoptilolites. Ind. Eng. Chem. Res. 30, 2523–2530 (1991)
Ackley, M.W., Giese, R.F., Yang, R.T.: Clinoptilolite: untapped potential for kinetic gas separations. Zeolites 12, 780–788 (1992)
Aguilar-Armenta, G., Hernandez-Ramirez, G., Flores-Loyola, E., Ugarte-Castaneda, A., Silva-Gonzalez, R., Tabares-Munoz, C., Jimenez-Lopez, A., Rodriguez-Castellon, E.: Adsorption kinetics of CO2, O2, N2, and CH4 in cation-exchanged clinoptilolite. J. Phys. Chem. B 105, 1313–1319 (2001)
Aguilar-Armenta, G., Patiño-Iglesias, M.E., Leyva-Ramos, R.: Adsorption kinetic behaviour of pure CO2, N2 and CH4 in natural clinoptilolite at different temperatures. Adsorpt. Sci. Technol. 21, 81–91 (2003)
Aguilar-Armenta, G., Romero-Perez, A.: Adsorption of C2H4, C2H6 and CO2 on cation-exchanged clinoptilolite. Adsorpt. Sci. Technol. 27, 523–536 (2009)
Ahmed, M.J., Theydan, S.K.: Equilibrium isotherms and adsorption heats analysis for ternary mixture of methane, ethane, and propane on 4A zeolite. J. Porous Mater. 21, 747–755 (2014)
Antoniou, M.K., Diamanti, E.K., Enotiadis, A., Policicchio, A., Dimos, K., Ciuchi, F., Maccallini, E., Gournis, D., Agostino, R.G.: Methane storage in zeolite-like carbon materials. Microporous Mesoporous Mater 188, 16–22 (2014)
Arcoya, A., Gonzalez, J.A., Travieso, N., Seoane, X.L.: Physicochemical and catalytic properties of a modified natural clinoptilolite. Clay Miner. 29(1), 123–131 (1994)
Arcoya, A., González, J.A., Llabre, G., Seoane, X.L., Travieso, N.: Role of the countercations on the molecular sieve properties of a clinoptilolite. Microporous Mater. 7(1), 1–13 (1996)
Barrer, R.M.: Zeolites and Clay Minerals as Sorbents and Molecular Sieves. Academic Press, New York (1978)
Berlier, K., Olivier, M.G., Jadot, R.: Adsorption of methane, ethane, and ethylene on zeolite. J. Chem. Eng. Data 40, 1206–1208 (1995)
Breck, D.W.: Zeolite Molecular Sieves. Wiley, New York (1984)
Brunauer, S., Deming, L.S., Deming, W.E., Teller, E.: On a theory of the van der Waals adsorption of gases. J. Am. Chem. Soc. 62, 1723–1732 (1940)
Cardarelli, F.: Materials Handbook: A Concise Desktop Reference, 2nd edn. Springer, New York (2008)
Carson, P., Mumford, C.: Hazardous Chemicals Handbook, 2nd edn. Butterworth-Heinemann, Burlington (2002)
Cavenati, S., Grande, C.A., Rodrigues, A.E.: Adsorption equilibrium of methane, carbon dioxide, and nitrogen on zeolite 13X at high pressures. J. Chem. Eng. Data 49, 1095–1101 (2004)
Christidis, G.E., Moraetis, D., Keheyan, E., Akhalbedashvili, L., Kekelidze, N., Gevorkyan, R., Yeritsyan, H., Sargsyan, H.: Chemical and thermal modification of natural HEU-type zeolitic materials from Armenia, Georgia and Greece. Appl. Clay Sci. 24(1–2), 79–91 (2003)
Choudhary, V.R., Mayadevi, S., Pal Singh, A.: Sorption isotherms of methane, ethane, ethene and carbon dioxide on NaX, NaY and Na-mordenite zeolites. J. Chem. Soc., Faraday Trans. 91, 2935–2944 (1995)
Çakıcıoğlu-Özkan, F., Ülkü, S.: The effect of HCl treatment on water vapor adsorption characteristics of clinoptilolite rich natural zeolite. Microporous Mesoporous Mater. 77, 47–53 (2005)
Ding, T.F., Ozawa, S., Yamazaki, T., Watanuki, I., Ogino, Y.: A generalized treatment of adsorption of methane onto various zeolites. Langmuir 4, 392–396 (1988)
Dyer, A.: An Introduction to Zeolite Molecular Sieves. Wiley, New York (1988)
Esenli, F., Sirkecioğlu, A.: The relationship between zeolite (heulandite–clinoptilolite) content and the ammonium-exchange capacity of pyrodastic rocks in Gordes, Turkey. Clay Miner. 40(4), 557–564 (2005)
Galli, E., Gottardi, G., Mayer, H., Preisinger, A., Passaglia, E.: The structure of potassium-exchanged heulandite at 293, 373 and 593 K. Acta Crystallogr. B 39, 189–197 (1983)
Garcia-Basabe, Y., Rodriguez-Iznaga, I., de Menorval, L., Llewellyn, P., Maurin, G., Lewisf, D.W., Binionsf, R., Autieg, M.A., Ruiz-Salvadora, R.: Step-wise dealumination of natural clinoptilolite: structural and physicochemical characterization. Microporous Mesoporous Mater. 135(1–3), 187–196 (2010)
Gottardi, G., Galli, E.: Natural Zeolites. Springer, Berlin (1985)
Grande, C.A., Blom, R.: Cryogenic adsorption of methane and carbon dioxide on zeolites 4A and 13X. Energy Fuels 28, 6688–6693 (2014)
Gregg, S.J., Sing, K.S.W.: Adsorption, Surface Area and Porosity, 2nd edn. Academic Press, London (1982)
Hamzah, S.A., Mahmood, N.Z., Sulaiman, A.H.: Ozone depletion substances (ODS) emission analysis from the life cycle of chemical substances and electricity used in potable water production in Malaysia. Aust. J. Basic & Appl. Sci. 4 (9), 4286–4293 (2010)
Hernandez-Beltran, N.A., Olguin, M.T.: Elemental composition variability of clinoptilolite-rich tuff after the treatment with acid phosphate solutions. Hydrometallurgy 89, 374–378 (2007)
Hernández-Huesca, R., Díaz, L., Aguilar-Armenta, G.: Adsorption equilibria and kinetics of CO2, CH4 and N2 in natural zeolites. Sep. Purif. Technol. 15, 163–173 (1999)
Jayaraman, A., Yang, R.T., Chinn, D., Munson, C.L.: Tailored clinoptilolites for nitrogen/methane separation. Ind. Eng. Chem. Res. 44, 5184–5192 (2005)
Korkuna, O., Leboda, R., Skubiszewska-Zeiba, J., Vrublevska, T., Gunko, V.M., Ryczkowski, J.: Structural and physicochemical properties of natural zeolites: clinoptilolite and mordenite. Microporous Mesoporous Mater. 87, 243–254 (2006)
Kouvelosa, E., Kesoreb, K., Steriotisa, T., Grigoropoulouc, H., Bouloubasid, D., Theophiloud, N., Tzintzosd, S., Kanelopoulosa, N.: High pressure N2/CH4 adsorption measurements in clinoptilolites. Microporous Mesoporous Mater. 99, 106–111 (2007)
Koyama, K., Takeuchi, Y.: Clinoptilolite: the distribution of potassium atoms and its role in thermal analysis. Z. Kristallogr. 145, 216–239 (1977)
Lide, D.R.: CRC Handbook of Chemistry and Physics. CRC Press, Boca Raton (2003)
Macedonia, M.D., Moore, D.D., Maginn, E.J., Olken, M.M.: Adsorption studies of methane, ethane, and argon in the zeolite mordenite: molecular simulations and experiments. Langmuir 16, 3823–3834 (2000)
Maple, M.J., Williams, C.D.: Separating nitrogen/methane on zeolite like molecular sieves. Microporous Mesoporous Mater. 111, 627–631 (2008)
Merkle, A.B., Slaughter, M.: Determination and refinement of the structure of heulandite. Am. Mineral. 53, 1120–1138 (1968)
Moore, D.M., Reynolds Jr, R.C.: X-ray Diffraction and the Identification and Analysis of Clay Minerals, 2nd edn. Oxford University Press, New York (1997)
Mumpton, F.A.: Clinoptilolite redefined. Am. Mineral. 45, 351–369 (1960)
Patnaik, P.: Handbook of Environmental Analysis, Chemical Pollutants in Air, Water, Soil, and Solid Wastes, 2nd edn. CRC Press, Boca Raton (2010)
Petrakakis, Y., Mylona, E., Georgantas, D., Grigoropoulou, H.: Leaching of lead from clinoptilolite at acidic conditions. Global NEST J. 9(3), 207–213 (2007)
Predescu, L., Tezel, F.H., Stelmack, P.: Adsorption of Nitrogen and Methane on Natural Clinoptilolite. In: Bonneviot, L., Kaliaguin, S. (eds.) Zeolites: A Refined Tool for Designing Catalytic Sites, pp. 507–512. Elsevier, Amsterdam (1995)
Radosavljević-Mihajlović, A., Dondur, V., Daković, A., Lemić, J., Tomašević-Čanović, M.: Physicochemical and structural characteristics of HEU-type zeolitic tuff treated by hydrochloric acid. J. Serb. Chem. Soc. 69(4), 273–281 (2004)
Rozic, M., Cerjan-Stefanovic, S., Kurajica, S., Rozmari Maeefat, M., Margeta, K., Farkas, A.: Decationization and dealumination of clinoptilolite tuff and ammonium exchange on acid-modified tuff. J. Colloid Interface Sci. 284, 48–56 (2005)
Salvestrini, S., Sagliano, P., Iovino, P., Capasso, S., Colella, C.: Atrazine adsorption by acid activated zeolite-rich tuffs. Appl. Clay Sci. 49, 330–335 (2010)
Shindell, D.T., Faluvegi, G., Koch, D.M., Schmidt, G.A., Unger, N., Bauer, S.E.: Improved attribution of climate forcing to emissions. Science 326(5953), 716–718 (2009)
Triebe, R.W., Tezel, F.H., Khulbe, K.C.: Adsorption of methane, ethane and ethylene on molecular sieve zeolites. Gas Sep. Purif. 10, 81–84 (1996)
Tsitsishvili, G.V.: Physicochemical properties of high silica L and clinoptilolite zeolites. In: Meier, W.M., Uytterhoeven, J.B. (eds.) Molecular Sieves, pp. 291–298. ACS, Washington DC (1973)
Acknowledgments
Special thanks to Dr. Matthias Thommes for his helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alver, B.E., Sakizci, M. Influence of acid treatment on structure of clinoptilolite tuff and its adsorption of methane. Adsorption 21, 391–399 (2015). https://doi.org/10.1007/s10450-015-9679-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-015-9679-3