Abstract
Thermal analysis (thermogravimetry and differential thermal analysis) was used with scanning electron microscopy technique to investigate the hydration mechanisms and the microstructure of Portland cement-Fly ash-silica fume mixes. Calcium silicate hydrate (C–S–H), ettringite, gehlenite hydrate (C2ASH8), calcium hydroxide (Ca(OH)2) and calcium carbonate (CaCO3) phases were detected in all mixes. In the mixes with the use of silica fume addition, there is a decrease in Ca(OH)2 with increasing silica fume content at 5 and 10% compared to that of the reference Portland-fly ash cement paste and a corresponding increase in calcium silicate hydrate (C–S–H).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fly ash, a by-product from coal power plants, has been recognized as an important construction material due to its environmental benefits and engineering benefits (produce less heat of hydration, increase workability and improve durability to chemical attacks such as chlorides and sulphates) and has been used extensively world wide as a cement replacement material [1, 2]. However, it is commonly known that fly ash, which is a pozzolanic material i.e. will react to form a binding material similar to that of Portland cement in the presence of water and calcium hydroxide, Ca(OH)2 [2–4]. This pozzolanic reaction with Ca(OH)2 occurs slowly due to Ca(OH)2 has to be first produced as a result of hydration product of Portland cement (PC) and also that the particle size of normally usable fly ash is somewhat coarser than that of PC. The particle size of typical fly ash used is within the region of 66–100% passing at 45 μm or not more than 34% retaining at this sieve size according to ASTM C618 [5] as compared to the average particle size of PC at ≈ 10–15 μm.
Silica fume occurred as a by-product of the ferro-silicon alloys and silicon metal industries, on the other hand, is very fine at the average particle size of ≈100 nm [1]. Its high reactivity is known to benefit the strength of concrete where a significant gain in strength of concrete has been reported [6–10] when used on its own as a cement replacement material in its own right. The use of silica fume as an additional material in fly ash blended cement such as Portland cement-Fly ash-silica fume mix has recently attracted interests in the research of its effects on properties of fly-ash mixes, mainly in term of its strength and durability benefits for use as a construction material [11–13]. However, the hydration study of this mix in the paste system is limited [14, 15]. For the first 24 h the hydration of Portland cement-Fly ash silica fume paste has been reported to show that there is a reduction in Ca(OH)2 content [14]. Little is known however, in terms of the effect thereafter and that no full investigation of the system have been found using both thermogravimetric and differential thermal analysis. In this work, fly ash (FA), was blended with Portland cement (PC) to produce Portland-Fly ash cement pastes and silica fume (SF), as a highly reactive material was then used to produce ternary cement paste mixes (Portland cement-Fly ash-silica fume). Thermal analysis in terms of thermogravimetric and differential thermal analysis were then used with scanning electron microscopy technique to investigate the hydration mechanisms and the microstructure of Portland cement-Fly ash-silica fume mixes. Silica fume was used as an additional material at 5 and 10 wt% to the Portland cement-fly ash mixes. As part of this investigation compressive strength studies of the ternary blends were also carried out to correspond with the thermal analysis in order to determine hydration products of Portland cement-fly ash-silica fume pastes with further investigation on the microstructure of the samples were also carried out.
Experimental techniques
Portland cement type I (PC) was used in the investigation. In this study, fly ash (FA) was obtained from Mae Mao Power Plant, Lampang, Thailand and silica fume (SF) was obtained from Sikament Ltd. Chemical composition of these materials are given in Table 1. The mixes of Portland cement-Fly ash-silica fume pastes are given in Table 2. Fly ash was used as a Portland cement replacement at 10, 20 and 30 wt% to produce Portland-fly ash cement and silica fume was used as an additive material which was added at 5 and 10 wt% to Portland-fly ash cement. The water/binder ratio of these cement pastes used was 0.5.
To increase the homogeneity, the binders (PC, FA and SF) were first mixed together in the mixer for 3 min, and then the water was added. The mixture was mixed for another 2 min. After that, the mixes were poured into oiled molds (50 × 50 × 50 mm3) and then compacted. The specimens were then surface-smoothed, and covered with plastic film. All specimens were removed from the moulds 1 day after casting. Thereafter, they were cured in water at room (≈25 °C) temperature. For compressive strength tests of the pastes, fly ash was used as a cement replacement at 10, 20 and 30 wt% with and without silica fume addition. Compressive strength tests were carried out using three specimens for each test and an average result was then obtained at the age of 7, 28 and 90 days.
Also at 28 days, selective samples of mainly 20% fly ash were used to represent typical sample of the mixes with and without silica fume for microstructure and thermal analyses. The microstructure of the samples was investigated using scanning electron microscopy (SEM; JEOL-JSM-840A) and then ground for thermal analysis (thermogravimetry and differential thermal analysis) using the Mettler Toledo TG/SDTA 851e. The samples were heated from room temperature to 1,000 °C with a scanning rate of 10 °C min−1 under nitrogen atmosphere condition. In addition, thermogravimetry and differential thermal analyses were also carried out for Portland cement-fly ash (20%)-silica fume (10%) sample at 7, 28 and 90 days in order to determine the effect of time on the hydration phases of the ternary mix.
Results and discussion
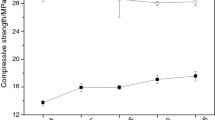
The influence of silica fume on the compressive strengths of the Portland-fly ash cement pastes at 28 days is shown in Fig. 1a. The results show for all 10, 20 and 30 wt% of fly ash content without silica fume content, the values of compressive strength of fly ash mixes (37–45 MPa) are all less than those of Portland cement paste (47 MPa) as can be seen from Fig. 1a. However, when silica fume was added (Portland cement-fly ash-silica fume pastes), the compressive strength was found to increase significantly with the use of silica fume in the mix. In some cases (10FA5SF and 10FA10SF) the compressive strengths (48.5 and 50 MPa, respectively) were higher than Portland cement paste. For 20% fly ash mix with silica fume with 10% addition of silica fume (20FA10SF) the compressive strengths were very close to that of Portland cement paste at 28 days and become just slightly higher at 90 days as shown in Fig. 1b (57.0 MPa compare to control PC of 56.5 MPa). It also remained to be significantly higher than the reference fly ash mix without silica fume where the compressive strength was at 53.0 MPa. The enhancement in the strength was expected to be due to the pozzolanic reaction of silica fume where thermal analysis is required in order to confirm this pozzolanic reaction has taken placed. This is described in the following section.
From X-ray diffraction traces of the Portland cement-fly ash-silica fume pastes (Fig. 2), dominant peaks detected by means of X-ray diffraction (XRD) was calcium hydroxide Ca(OH)2 noted as CH. The Ca(OH)2 peaks can be seen in all mixes, thus this give an idea of the hydration reaction of each mixes. As can be seen from XRD traces, it is clear that for the fly ash mix with the use of silica fume addition, the intensity of Ca(OH)2 is less than that of the reference fly ash mix without silica fume. This shows that the pozzolanic reaction (SiO2 and/or Al2O3 reaction with Ca(OH)2) [2–4] has taken placed, whereby Ca(OH)2 was being clearly consumed by silica fume.
Thermogravimetric analysis (TG) was then used to further analyse the phases which do not appear by the use of XRD technique which only shows the crystalline phases. The results obtained from TG, as shown in Fig. 3, for Portland cement and Portland-Fly ash cement pastes with and without the addition of SF (using FA at 20% to represent typical effect of SF) shows three mass loss transition with the first being from room temperature ≈30–420 °C, the second from ≈420–500 °C and the third ≈500–1,000 °C. TG can be used to determine quantitatively from the mass loss of the liquid (H2O) or gas (CO2) of the hydrated phases. The phases detected by means of TG at the first transition which consist of several hydrated phases at this range which are ettringite, C–S–H and C2ASH8. The second transition which occurred from the (dehydroxilation) of water from Ca(OH)2, and the third transition of mass loss occurred from the decarbonation of calcium carbonate.
The mass loss of the pastes of each phases can be calculated from each transition occurring in the TG curves and also can be further calculated to give the relative mass loss if taken overall mass loss as 100%. The calculated values for Portland cement, fly ash mixes with and without silica fume are given in Table 3, where the relative mass loss from all transitions can be seen as both the mass loss of the sample and also as mass loss to overall mass loss. For these calculations, a selective mixes of fly ash with silica fume were selected i.e. using 10% fly ash with 5% SF and 20% fly ash with both 5 and 10% SF as representatives. For the case without SF, the first transition which covers the mass loss of the hydrated phases (ettringite, C–S–H and C2ASH8) between room temperature to 420 °C was found to be highest in Portland cement paste (21.10%). For the reference Portland cement-fly ash mix without silica fume, this mass loss was found to be less (at 20.35% for 10% FA mix and 19.90% for 20% FA mix) due to less amount of the C–S–H phase which would result in lower strength as found. On the other hand this mass loss was found to increase slightly with the addition of silica fume at 5% (21.63 and 19.97% for 10% FA mix and 20% FA mix respectively). Moreover, it is clear from the second phase that calcium hydroxide can be seen to reduce when FA is used (3.44% for 20% FA with no SF) and is further reduced when SF is added (3.02% for 5% SF and 2.56% for 10% SF) when compared to the control PC paste at 4.80%. Further analysis is then carried out in term of overall mass loss, the mass loss in each transition to overall mass loss (i.e. overall mass loss is taken as 100%) for Portland cement-fly ash-silica fume mixes using fly ash at 20% with and without SF to represent a typical effect of SF, is shown (inset) in Fig. 3. An increase in the mass loss in the first transition attributed to ettringite, C–S–H and C2ASH8 with respect to SF content can be seen and as a consequent there is a decrease in the second transition attributed to Ca(OH)2 due to the pozzolanic reaction (consumption of Ca(OH)2 with SF).
Nonetheless, the mass loss in the first transition cannot be used to single out an individual phase such as C–S–H since the range of mass loss in each transition is rather broad and therefore using TG alone can not established this. Derivative thermogravimetric (DTG) and differential thermal analysis (DTA) therefore, were used to further determine each phases. The DTG results of Portland cement pastes and of Portland cement-Fly ash-silica fume pastes are plotted as shown in Fig. 4a. After 28 days curing in water the DTG curves for all pastes show detected phases which are calcium silicate hydrate (C–S–H), 6CaO·Al2O3·3SO3·32H2O (ettringite), 2CaO·Al2O3·SiO2·8H2O or gehlenite hydrate (noted as C2ASH8), calcium hydroxide (notes as CH for convenience) and calcium carbonate (CaCO3, noted as CC) detected at ≈76–84 °C, ≈104–114 °C, ≈157–163 °C, ≈458–464 °C and ≈667–688 °C, respectively. The comparison of DTG graphs of Portland-fly ash cement pastes with and without silica fume (curing in water at 28 days) when replaced Portland cement with fly ash at 20 wt% and added silica fume up to 10 wt% are presented. Although, the phases detected were found to be similar to those in Portland cement paste, it is noticed from the DTG curves (Fig. 4a) that there is a decrease in Ca(OH)2 with increasing silica fume content at 5 and 10% compared to that of the reference Portland-fly ash cement paste. This was due to the increase in pozzolanic reaction when silica fume was also added thus consuming more of calcium hydroxide. Consequently, it is evidently clear that more of calcium silicate hydrate (C–S–H) can be detected in both mixes with silica fume and this is seen increasing with silica fume content. This increase in C–S–H would therefore agree with the compressive strength results where the compressive strength also increases with SF content.
DTA curves of hydrated Portland cement-fly ash-silica fume samples using fly ash at 20% samples with and without SF is shown plotted in Fig. 4b. Three main endothermic peaks were detected as can be seen from these DTA curves. The first endothermic peak in the temperature range of 82–163 °C is related to the dehydration of calcium silicates and calcium alumino silicate hydrates. Furthermore, this peak can be noticed to be stronger in intensity for mixes with SF. The second endothermic peak can be seen at the temperature range of 455–466 °C which is attributed to the Ca(OH)2 decomposition. The third endothermic peak can be seen at 697–724 °C as a result of CaCO3 decomposition. These temperature ranges for the three main peaks detected are in similar to the ones detected in DTA curves by Giergiczny [16] and Amer [17].
In addition, TG was used to study the effect of time on the mass loss of the Portland cement-fly ash (20%)-silica fume (10%) sample at 7, 28 and 90 days and these results are shown in Fig. 5. The mass loss can be seen to be greater with time and that the over mass loss is also greater with increasing period of curing. Again, the mass loss can be calculated as to the overall mass of the cement paste samples and also as relative percentages to the overall mass loss of 100%, and the calculations of these mass loss at three main transitions are given in Table 4. It can be seen that mass the loss in the first transition increased when the curing period was increased thus producing more hydration products with time. As a consequent, silica fume would consumed more Ca(OH)2 and thus this reduction is seen. These trends are also clearly seen when plotted against time as relative percentages to overall mass loss percentages of three transitions as shown in the inset of Fig. 5.
The effect of curing time can also be further analysed with the use of DTG, where the DTG plots for this ternary mix (20% FA with 10% SF) at 90 days are compared to the results of 7 and 28 days as shown in Fig. 6a. Calculated mass loss of the phases in relation to these derivatives can also be seen in Table 4. The hydration products such as calcium silicate hydrate (C–S–H) can clearly be noticed to increase with time and that a reduction of Ca(OH)2 is seen as a direct consequent of pozzolanic reaction with time to produce the resulting C–S–H. This therefore explained the increase in the compressive strength of this ternary mix when the curing time was increased.
An increase in the endothermic peak for the hydration products is also evident in Fig. 6b. In a sense the endothermic peak of CaOH2 represented as CH, is weaker in sample of 90 days compared to the one at 28 days. Although, the endothermic peak of CaOH2 at 7 days is not the strongest, it is however by far the broadest when compared to the peaks at longer period. Therefore, DTA curves can be seen to show an increase in CSH but not clear for calcium hydroxide and calcium carbonate, this is not understood why. However, when also carefully considering with TG and DTG results that CaOH2 likely to be less as curing period increased.
The microstructure of Portland-fly ash cement pastes was analyzed using SEM. Figure 7a, b shows SEM micrographs of Portland-fly ash cement pastes with silica fume at 10%. Two fly ash particles can be seen with one part of a fly ash particle (spherical in shape) can be seen covered by C–S–H phase (Fig. 7a) which means that some of this fly ash already reacted with Ca(OH)2. This incomplete reaction, together with another fly ash particle still showing no sign of any reaction taken placed confirm that fly ash is a slow reactive material. Silica fume particles, on the other hand, would have already reacted to form hydration products as detected by DTG curve.
At a much higher magnification, parts of the C–S–H were seen to be covering silica fume particles and silica fume particles themselves also filled the pores between C–S–H and C2ASH8 phases (silica fume particles are shown using arrows in Fig. 7b) which give the matrix a denser microstructure, thus resulting to a higher strength gain.
Conclusions
Using thermal analysis to aid the explanation to the increase in compressive strength results of typical Portland cement-fly ash-silica fume pastes, calcium silicate hydrate (C–S–H), ettringite, gehlenite hydrate (C2ASH8), calcium hydroxide (CH) and calcium carbonate (CaCO3) phase were detected in all mixes. In the mixes with the use of silica fume addition, there is a decrease in Ca(OH)2 with increasing silica fume content at 5 and 10% compared to that of the reference Portland-fly ash cement paste and a corresponding increase in calcium silicate hydrate (C–S–H) can be detected. Using SEM, C–S–H phase can be seen in all Portland cement-fly ash mixes. Parts of the C–S–H were found to be covering silica fume particles and silica fume particles themselves also filled the pores between C–S–H phases which give the matrix a denser microstructure, thus resulting to a higher strength gain than the corresponding Portland-Fly ash cement paste without silica fume. It was found that the benefit of additional use of silica fume is clearly evident from the SEM micrographs where a significantly denser microstructure can be observed.
References
Malhotra VM, Mehta PK. Pozzolanic and cementitious materials, advance in concrete technology, vol. 1. USA: Gordon and Breach Publishers; 1996. p. 11.
Alonso JL, Wesche K. Characterization of fly ash. In: Wesche K, editor. Fly ash in concrete. London: E and FN SPON; 1991. p. 3.
Baert G, Hoste S, Schutter G, Belie N. Reactivity of fly ash in cement paste studied by means of thermogravimetry and isothermal calorimetry. J Therm Anal Calorim. 2008;94:485–92.
Vessalas K, Thomas PS, Ray AS, Guerbois JP, Joyce P, Haggman J. Pozzolanic reactivity of the supplementary cementitious material pitchstone fines by thermogravimetric analysis. J Therm Anal Calorim. 2009. doi:10.1007/s10973-008-9708-5
ASTM C618. Standard specification for fly ash and raw calcined natural pozzolan for use as a mineral admixture in Portland cement concrete. Philadelphia: The American Society for Testing Materials; 1991.
Khalil A. Pore structure and surface area of hardened cement pastes containing silica fume. Mat Lett. 1996;26:259–64.
Turkmen I, Gavgali M, Gul R. Influence of mineral admixtures on the mechanical properties and corrosion of steel embedded in high strength concrete. Mat Lett. 2003;57:2037–43.
Turkmen I, Gavgali M. Influence of mineral admixtures on the some properties and corrosion of steel embedded in sodium sulfate solution of concrete. Mat Lett. 2003;57:3222–33.
Chung DDL. Review: improving cement-based materials by using silica fume. J Mater Sci. 2002;37:673–82.
Zivica V. Effectiveness of new silica fume alkali activator. Cement Concr Comp. 2006;28:21–5.
Thomas MDA, Shehata MH, Shashiprakash SG, Hopkins DS, Cail K. Use of ternary cementitious systems containing silica fume and fly ash in concrete. Cem Concr Res. 1999;29:1207–14.
Langan BW, Weng K, Ward MA. Effect of silica fume and fly ash on heat of hydration of Portland cement. Cem Concr Res. 2002;32:1045–51.
Jaturapitakkul C, Kiattikomol K, Sata V, Leekeeratikul T. Use of ground coarse fly ash as a replacement of condensed silica fume in producing high-strength concrete. Cem Concr Res. 2004;34:549–55.
Temiz H, Karakeci AY. An investigation on microstructure of cement paste containing fly ash and silica fume. Cem Concr Res. 2002;32:1131–2.
Lilkov V, Dimitrova E, Petrov OE. Hydration process of cement containing fly ash and silica fume: the first 24 hours. Cem Concr Res. 1997;27:577–88.
Giergiczny Z. Effect of some additives on the reactions in fly ash-Ca(OH)2 system. J Therm Anal Calorim. 2004;76:747–54.
Amer AA. Thermal analysis of hydrated fly ash-lime pastes. J Therm Anal Calorim. 1998;54:837–43.
Acknowledgements
The authors gratefully acknowledge the Nano Science and Nanotechnology Center, Faculty of Science, Chiang Mai University, the Thailand Research Fund and the Commission for Higher Education for funding this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chaipanich, A., Nochaiya, T. Thermal analysis and microstructure of Portland cement-fly ash-silica fume pastes. J Therm Anal Calorim 99, 487–493 (2010). https://doi.org/10.1007/s10973-009-0403-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0403-y