Abstract
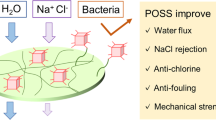
Organosilica-based reverse osmosis (RO) membranes are beneficial for water desalination, owing to their robust properties such as chlorine resistance and thermal stability. However, their water permeance is lower than that of conventional membranes. In a first, carboxyl-functionalized polyhedral oligomeric silsesquioxane (POSS–COOH) was utilized in this work as a hydrophilic nanofiller to improve the water permeance characteristic of organosilica-based RO membranes. The monomer, 1,2-Bis(triethoxysilyl)ethane (BTESE), was subjected to acidic hydrolytic polycondensation in the presence of POSS–COOH in various amounts to produce films and membranes. The water contact angle of the films decreased with increasing POSS–COOH content, indicating that POSS–COOH can work as a hydrophilic agent in the membrane. Scanning electron microscope observations of the membrane surface revealed the formation of linear patterns due to phase separation, which was caused by POSS–COOH aggregation with a large interaction of the carboxyl group. The composite membrane BTESE/POSS–COOH exhibited water desalination properties. Their water permeance increased significantly, while salt rejection decreased gradually, with increasing POSS–COOH concentration. This phenomenon arises from increased hydrophilicity and the phase separation structure of the membrane.

Highlights
-
Composite membranes composed of organosilica and POSS–COOH were prepared.
-
The organosilica sol with POSS–COOH was dispersed in ethanol.
-
The POSS–COOH region on the membrane aggregated and caused phase separation.
-
The contact angles of the films decreased with increasing POSS–COOH amount.
-
As POSS–COOH amount increased, water permeance increased & NaCl rejection decreased.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Reverse osmosis (RO) membranes represent an effective water purification technology utilized for ultrapure water production, wastewater purification, and seawater desalination [1, 2]. Thin-film composite membranes fabricated from polyamide (PA) [3], and cellulose acetate (CA) membranes with asymmetric porous structures [4] are widely used in water desalination applications because of their excellent water separation properties. However, these membranes suffer from a fouling process, which covers their surfaces with various contaminants such as biofilms [5]. Although washing the membrane surface with a chlorine-containing agent effectively removes the biofilm, the membrane often becomes damaged after this treatment due to the cleavage of polymer chains (especially in PA-based membranes). Therefore, chlorine-resistant membranes have been developed by many researchers. Recently, Tsuru et al. reported a bridged organosilica membrane (e.g., 1,2-Bis(triethoxysilyl)ethane, BTESE) with the general chemical formula [O1.5SiRSiO1.5]n and high chlorine resistance due to its inorganic siloxane framework. In addition, the presence of Si–C bonds in the organosilica structure resulted in high hydrothermal stability, which allowed membrane operation at temperatures as high as 90 °C [6]. Therefore, bridged polysilsesquioxane is a promising material for RO membranes. [7,8,9,10,11,12,13] However, the water permeance of such membranes is lower than that of PA and CA membranes.
To enhance the anti-fouling and water permeation properties of RO membranes, mixed matrix membranes, which represent composite membranes containing nanofiller components such as carbon nanotubes [14], SiO2 [15], TiO2 [16], graphene oxide [17], zeolites [18], and metal-organic frameworks [19] were developed. Recently, polyhedral oligomeric silsesquioxane (POSS) has attracted considerable attention as a nanofiller for water desalination membranes [20,21,22,23,24,25,26,27,28,29,30,31,32]. POSS is a nano-molecule with unique and fascinating properties, including strong functionalization ability, large free volume resulting from the bulky structure, high thermal and mechanical stabilities originating from the inorganic siloxane (Si–O) core, and high compatibility and dispersibility in a polymer matrix [23]. For example, Duan et al. reported that four different POSS/PA membrane types increased the water flux by up to 65% compared with that of the pristine PA membrane [20]. Liu et al. reported that POSS surface grafting enhanced the anti-chlorine and antibacterial properties of PA-based membranes [21]. Worthley et al. demonstrated that the membrane anchoring CA to POSS nanofiller considerably increased the water flux and compaction resistance of the produced composite membranes [22]. These studies intended to improve the PA and CA membranes; however, reforming organosilica membranes using POSS nanofillers has not been reported.
Recently, Kaneko et al. developed a novel synthesis procedure for POSS compounds containing carboxyl groups (POSS–COOH) [24, 25]. The chemical structures representing mixtures of 8, 10, and 12 oligomeric siloxane compounds are shown in Fig. 1. Since these compounds cannot be easily separated to obtain a single component, we utilized the oligomeric compounds as a mixture. POSS–COOH was prepared from 2-cyanoethyl(triethoxy)silane in an open system via a simple two-step process. Thus, POSS–COOH can serve an efficient nanofiller for water desalination membranes because of its high hydrophilicity derived from the presence of carboxyl groups and inorganic nature.
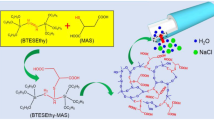
In this study, we employed POSS–COOH as a hydrophilic nanofiller for organosilica composite membranes. An organosilica precursor, BTESE, was used to prepare the organosilica membrane matrix. The main objective was to investigate the hydrophilicity and water permeability of RO membranes with various POSS–COOH contents. The utilized membrane preparation procedure is illustrated in Fig. 2. We found that the incorporation of POSS into the BTESE polymer matrix increased the hydrophilicity and water permeance of the porous membrane support. This paper describes the first study employing hydrophilic POSS–COOH nanofiller for water desalination membranes.
2 Experimental
2.1 General
Ethanol, 6 mol/L hydrochloric acid, and methyl orange were purchased from Kanto Chemical Co., Inc (Tokyo, Japan). All the reagents were used as received without purification. POSS–COOH was synthesized as described elsewhere [25, 26]. The molar ratio of the produced POSS–COOH particles (T8:T10:T12 = 18:67:15), determined by the peak intensity of 29Si NMR spectrum as shown in Fig. S1, was slightly different from that reported in the literature (T8:T10:T12 = 15:64:21).
The size distribution of the sol particles was measured by dynamic light scattering (DLS) on a Litesizer 500 analyzer (Anton Parr, Graz, Austria). The Fourier-transform infrared (FT–IR) spectra of the composite films coated on silicon wafers were recorded using an FT/IR-6100 spectrometer (JASCO, Inc., Tokyo, Japan) in the transmission mode. The water contact angles of the films spin-coated on glass plates were measured using an SImage Entry 5 (Excimer Inc, Kanagawa, Japan.) instrument. The scanning electron microscopy (SEM) images were obtained using a JSM-6500F microscope (JEOL Ltd., Tokyo, Japan). Confocal laser scanning microscopy (CLSM) observations were performed using a color 3D laser scanning microscope (VK-8510, Keyence Corporation, Osaka, Japan). The electron conductivity was measured using an EC-33B meter (Horiba, Ltd., Kyoto, Japan).
2.2 Sol preparation
BTESE with and without POSS–COOH (a total of 0.10 g) was dissolved in 1.66 g of ethanol and stirred for 10 min. HCl (0.1 eq.) and H2O (60 eq.) were slowly added to the prepared 5 wt% solution of BTESE and POSS–COOH in water and ethanol and stirred for 2 h at 25 °C. Subsequently, more ethanol was added to the solution to dilute it from 5 to 3 wt.%, which was then stirred for 10 min at 25 °C. The utilized BTESE/POSS–COOH weight ratios corresponding to the BTESE, BTPO-1, BTPO-5, BTPO-10, and BTPO-20 samples were 100/0, 99/1, 95/5, 90/10, and 80/20, respectively. The resulting mixture was stirred for 24 h to form a sol solution, which was stored at 4 °C.
2.3 Preparation of organosilica membranes
The obtained sol solution was poured through a 0.45 µm membrane filter onto a nanofiltration membrane (NTR-7430, Nitto Denko Corporation, Osaka, Japan) using a Teflon mold. After 30 s, the excess solution was air-flushed, and the membrane was dried at 150 °C for 10 min in air. The coating and drying processes were repeated twice. The resulting membrane was cut into a piece with a diameter of 25 mm to evaluate its RO performance. Then, BTPO-20 was immersed in the saturated methyl-orange aqueous solution for 10 min to investigate the existence of POSS–COOH.
2.4 Measurement of RO membrane
The water permeation setup with a dead-end system is schematically depicted in Fig. 3. The membrane is fixed by a stainless module filled with 2000 ppm NaCl aqueous solution. The module was pressurized for 15 h by nitrogen gas at a pressure of 1.5 MPa and a temperature of 25 °C due to water permeance. When the water permeance reached a steady state, the permeate solution was collected in four steps at ~2 h intervals. The water permeance (Lp) and NaCl rejection (R) were determined via the following equations.
where Wp, A, ΔP, Δπ, t, and Cp/Cf denote the weight of permeated water, area of the membrane, applied pressure, osmotic pressure, measurement time, and ratio of the NaCl concentration in the permeate solution to that in the feed solution, respectively. The membranes were tested several times, and the obtained data indicated high operational stability without significant performance reduction due to crack formation or other reasons.
3 Results and discussion
3.1 Properties of sol solution and thin film
Sol preparation was conducted under acidic conditions using diluted hydrochloric acid because acidic conditions make the organosilica sol have a polymer-like structure that is advantageous for preparing dense membranes, whereas alkali conditions make it particulate to form membranes with large pore [26]. Moreover, it is important that the carboxyl group does not react with acid catalysts. The BTESE and POSS–COOH components were mixed at weight ratios of 100:0, 99:1, 95:5, 90:10, and 80:20 to produce mixtures abbreviated as BTESE, BTPO-1, BTPO-5, BTPO-10, and BTPO-20, respectively. These mixtures were dissolved in ethanol and then subjected to hydrolysis and condensation procedures to obtain sol solutions. The sol particle sizes determined by DLS were ~1–2 nm (Fig. 4), which facilitates their coating on membrane supports, whose pore size is ~1.5 nm. These results indicate that the addition of POSS–COOH has no effect on the sol–gel reaction.
To confirm the presence of POSS–COOH species in the BTESE polymer matrix, composite thin films were prepared by drop casting on silicon wafers followed by heat treatment at 150 °C for 10 min. The FT–IR spectra of the produced films (Fig. 5) show that the intensity of the band corresponding to the stretching vibration of the carboxyl group (1704 cm−1, vC=O) increased upon increasing the POSS–COOH content [25]. When the carboxyl group interacts with the Si–OH group (generated by the hydrolysis of Si–OEt) to form a hydrogen bond, the band position shifts to a lower wavenumber [27, 28]. Because no band shifts were observed in the spectra, we can conclude that no interactions occurred between the carboxyl and Si–OH groups; moreover, the Si–OH group was not observed. The intensity of the band centered at 1117 cm−1, which is a characteristic of the stretching vibration of the POSS siloxane group, also increased with increasing POSS–COOH concentration, suggesting the incorporation of the entire cage structure into the polymer matrix without decomposition. The peaks due to BTESE species containing C–H (2980 cm−1), Si–C (1270 cm−1), and Si−OSi (1030 cm−1) groups were observed as well [29].
In general, the higher the hydrophilicity of a surface, the stronger is its water permeation ability because of the increase in the water affinity to the membrane surface and interpore region [30]. To investigate the hydrophilicity of the prepared membranes, the films coated onto glass plates were heated to 150 °C for 10 min. The measured water contact angles of the film surfaces are plotted in Fig. 6. It shows that the contact angle decreases with increasing POSS content, suggesting that the water affinity of the membrane surface would be enhanced by the carboxyl groups of the POSS−COOH species, which increase the water permeance.
Next, the sol solution was diluted to 3 wt% and coated onto nanoporous membrane supports (NTR-7430) followed by heating to 150 °C for 10 min to obtain RO membranes. The heating temperature was set to 150 °C because the organic polymer substrate can withstand up to 150 °C and it is known that the higher the heat temperature, the higher the salt rejection, while the water permeance decreases [11, 26]. The membranes were cut to circular pieces with the diameter of 25 mm for water desalination measurements.
The surface morphologies of the fabricated RO membranes were observed by SEM and optical microscopy (Fig. 7). The SEM image of the BTESE membrane showed no distinctive regions except for small dots, which may be attributed to BTESE polymer particles. Meanwhile, the POSS–COOH species are displayed as white whiskers. With increasing POSS content, the white area expanded, leading to phase separation. These phenomena occurred because of the aggregation of POSS–COOH particles caused by strong interactions between their carboxyl groups. Because the sol solution was uniformly dispersed in ethanol during DLS analysis, this aggregation might have occurred when the coating solution was drying on the substrates. To confirm that the whisker-like regions are due to the POSS-COOH phase, the membrane was immersed in an aqueous solution of methyl orange. Because the scales of the BTPO-20 images in Fig. 7a, b are very similar, the orange region shown in Fig. 7b corresponds to POSS–COOH species. In addition, cross-sectional SEM and CLSM images of the prepared membranes were obtained (Figs. S2–7). The thickness of the separation layer could not be estimated because of the softness of the polymer membrane. According to the CLSM data, the BTPO-20 membrane has a rougher surface than the other membranes produced in this study—furthermore, this corresponds to the morphologies observed in the SEM images.
3.2 Evaluation of RO membrane
The water desalination properties of the fabricated membranes were evaluated as well. As shown in Fig. 8, the studied membranes exhibited improved water desalination properties. With increasing the POSS–COOH concentration, the water permeance of the composite membranes increased. In particular, the water permeance of the BTPO-20 membrane was seven times higher than that of the pristine BTESE membrane, while its salt rejection decreased from 93.3 to 83.7%. Water permeance and NaCl rejection typically exhibit a trade-off relationship, which was also observed for the composite membranes in this study. BT-PO1 membrane. The BT-BO-1 membrane showed approximately four times higher water permeance than the BTESE membrane, indicating that the addition of a small amount of POSS-COOH was effective in increasing the water while holding the NaCl rejection. The NaCl rejection of the BTPO-5 membrane at ~90%, indicating its potential applicability as an RO membrane. Such a drastic increase in the water permeance with increasing POSS–COOH concentration was likely caused by the separation of the BTESE and POSS phases. The utilized solution may permeate the POSS–COOH region with poor desalination properties due to its lower density than that of the polymer matrix. According to the contact angle measurement data, POSS–COOH addition clearly increased the membrane hydrophilicity, which contributed to enhanced water permeation properties.
The separation characteristics of various membranes (including PA-based and CA-based membranes) are listed in Table 1. Here, the water permeances were calculated from the literature values of the water flux. This table shows that the water permeances of the PA and PA-based composite membranes are ~100 times higher than those of the BTESE and BTPO membranes because of the large surface area of the crimped structure and the high hydrophilicity of the PA-based membrane. Meanwhile, the parameters of the BTPO membranes are close to those of the CA-based membranes.
Because POSS–COOH is soluble in water to some extent, the POSS–COOH components would dissolve in water and permeate the membrane during RO operation, causing membrane degradation. Thus, a long-term operation test was conducted to check whether the POSS–COOH components in the BTESE matrix leach out. The BTPO-1 composite membrane, which has a high NaCl rejection (more than 90%), was used in the desalination test conducted for 120 h, and the results are presented in Fig. 9. The results show that the water permeance and NaCl rejection remained unchanged, indicating that leaching did not occur. In our previous study, we prepared organosilica membranes with Si–O–C bonds from hydroxymethyl(triethoxy)silane and conducted long-time RO experiments on them [32]. In the membranes, water permeance gradually increased from 7.3 × 10−13 m3/(m2 Pa s) to 4.0 × 10−12 m3/(m2 Pa s) and NaCl rejection gradually decreased from 87 to 50% after 50 h of RO operation, which are attributed to the hydrolysis of the Si–O–C bonds, leading to degradation of the membrane. Nonetheless, these phenomena were not observed in the BTPO-1 membrane. These results demonstrate that the rigid polymer matrix derived from BTESE retains the POSS–COOH components in the BTESE matrix to endure a long-term operation.
Chlorine resistance test was conducted to explore the robustness of the membrane. The BTPO-1 membrane, which showed favorable water separation ability, was contacted with a 100 ppm NaOCl solution. Afterwards, the separation performance was conducted. The operations were repeated every ~20 h. As shown in Fig. 10, no obvious changes were observed to the exposure time of 98 h (chlorine exposure = 9800 ppm h), indicating the membrane has high tolerance towards chlorine exposure. This result indicates that the membrane has the potential to clean the membrane surface up to the fouling substate.
4 Conclusions
The ethane-bridged organosilica membrane and carboxyl-functionalized cage silsesquioxanes (POSS–COOH) composite membranes were prepared for the first time, and their water desalination properties were evaluated. During membrane fabrication, the POSS–COOH species were incorporated into the organosilica matrix. The hydrophilicity and water permeance of the resulting composite films increased, while their NaCl rejection decreased with increasing POSS–COOH content. The obtained SEM images and results of water permeation measurements indicated the occurrence of phase separation in the membrane structure, leading to a drastic increase in water permeance and decreased salt rejection. The BTPO-1 membrane, 1 wt% addition of POSS-COOH to the BTESE, increased the water permeance while maintaining the NaCl rejection. To fabricate a membrane with desirable water separation characteristics, the BTESE and POSS–COOH phases should be more homogeneous, which will be explored in future studies. We believe that POSS–COOH nanofillers can be applied to a variety of membranes to improve water permeability.
References
Jiang S, Li Y, Ladewig BP (2017) A review of reverse osmosis membrane fouling and control strategies. Sci Total Environ 595:567–583. https://doi.org/10.1016/j.scitotenv.2017.03.235
Li Y, Li M, Xiao K, Huang X (2020) Reverse osmosis membrane autopsy in coal chemical wastewater treatment: evidences of spatially heterogeneous fouling and organic-inorganic synergistic effect. J Clean Prod 246:118964. https://doi.org/10.1016/j.jclepro.2019.118964
Habib S, Weinman ST (2021) A review on the synthesis of fully aromatic polyamide reverse osmosis membranes. Desalination 502:114939. https://doi.org/10.1016/j.desal.2021.114939
Idris A, Ismail AF, Noordin MY, Shilton SJ (2002) Optimization of cellulose acetate hollow fiber reverse osmosis membrane production using Taguchi method. J Membr Sci 205:223–237. https://doi.org/10.1016/S0376-7388(02)00116-3
Baker JS, Dudley LY (1998) Biofouling in membrane systems—a review. Desalination 118:81–89. https://doi.org/10.1016/S0011-9164(98)00091-5
Xu R, Wang J, Kanezashi M, Yoshioka T, Tsuru T (2011) Development of robust organosilica membranes for reverse osmosis. Langmuir 27:13996–13999. https://doi.org/10.1021/la203711u
Ibrahim SM, Nagasawa H, Kanezashi M, Tsuru T (2015) Robust organosilica membranes for high temperature reverse osmosis (RO) application: membrane preparation, separation characteristics of solutes and membrane regeneration. J Membr Sci 493:515–523. https://doi.org/10.1016/j.memsci.2015.06.060
Ibrahim SM, Nagasawa H, Kanezashi M, Tsuru T (2017) Organosilica bis(triethoxysilyl)ethane (BTESE) membranes for gas permeation (GS) and reverse osmosis (RO): the effect of preparation conditions on structure, and the correlation between gas and liquid permeation properties. J Membr Sci 526:242–251. https://doi.org/10.1016/j.memsci.2015.06.060
Ibrahim SM, Nagasawa H, Kanezashi M, Tsuru T (2020) Chemical-free cleaning of fouled reverse osmosis (RO) membranes derived from bis(triethoxysilyl)ethane (BTESE). J Membr Sci 601:117919. https://doi.org/10.1016/j.memsci.2020.117919
Xu R, Wang J, Kanezashi M, Yoshioka T, Tsuru T (2013) Reverse osmosis performance of organosilica membranes and comparison with the pervaporation and gas permeation properties. AIChE J 59:1298–1307. https://doi.org/10.1002/aic.13885
Yamamoto K, Saito I, Amaike Y, Nakaya T, Ohshita J, Gunji T (2020) Preparation and water desalination properties of bridged polysilsesquioxane membranes with divinylbenzene and divinylpyridine units. Polym J 52:1367–1374. https://doi.org/10.1038/s41428-020-0386-x
Yamamoto K, Muragishi H, Mizumo T, Gunji T, Kanezashi M, Tsuru T, Ohshita J (2018) Diethylenedioxane-bridged microporous organosilica membrane for gas and water separation. Sep Purif Technol 207:370–376. https://doi.org/10.1016/j.seppur.2018.06.036
Yamamoto K, Koge S, Sasahara K, Mizumo T, Kaneko Y, Kanezashi M, Tsuru T, Ohshita J (2017) Preparation of bridged polysilsesquioxane membranes from bis[3-(triethoxysilyl)propyl]amine for water desalination. Bull Chem Soc Jpn 90:1035–1040
Kim HJ, Lim MY, Jung KH, Kim DG, Lee JC (2015) High-performance reverse osmosis nanocomposite membranes containing the mixture of carbon nanotubes and graphene oxides. J Mater Chem A 13:36798–6809. https://doi.org/10.1039/C4TA06080F
Shen H, Wang S, Xu H, Zhou Y, Gao C (2018) Preparation of polyamide thin film nanocomposite membranes containing silica nanoparticles via an in-situ polymerization of SiCl4 in organic solution. J Membr Sci 565:145–156. https://doi.org/10.1016/j.memsci.2018.08.016
Jeon S, Lee J-Y (2020) Rationally designed in-situ fabrication of thin film nanocomposite membranes with enhanced desalination and anti-biofouling performance. J Membr Sci 615:118542. https://doi.org/10.1016/j.memsci.2020.118542
Liu Y, Tan J, Choi W, Hsu J-H, Han DS, Han A, Abdel-Wahab A, Yu C (2018) Influence of nanoparticle inclusions on the performance of reverse osmosis membranes. Environ Sci Water Res Technol 4:411–420. https://doi.org/10.1039/C7EW00420F
Fathizadeh M, Aroujalian A, Raisi A (2011) Effect of added NaX nano-zeolite into polyamide as a top thin layer of membrane on water flux and salt rejection in a reverse osmosis process. J Membr Sci 375:88–95. https://doi.org/10.1016/j.memsci.2011.03.017
Duan J, Pan Y, Pacheco F, Litwiller E, Lai Z, Pinnau I (2015) High-performance polyamide thin-film-nanocomposite reverse osmosis membranes containing hydrophobic zeolitic imidazolate framework-8. J Membr Sci 476:303–310. https://doi.org/10.1016/j.memsci.2014.11.038
Duan J, Litwiller E, Pinnau I (2015) Preparation and water desalination properties of POSS-polyamide nanocomposite reverse osmosis membranes. J Membr Sci 473:157–164. https://doi.org/10.1016/j.memsci.2014.09.022
Liu Y, Liu C, Fu X, Lin O, Wang Z, Wang C, Zhang C (2019) Armor polyamide reverse osmosis membrane with POSS ‘armors’ through two-step interfacial polymerization for high anti-chlorine and anti-bacteria performance. J Membr Sci 586:211–221. https://doi.org/10.1016/j.memsci.2019.05.052
Worthley CH, Constantopoulos KT, Ginic-Markovic M, Markovic E, Clarke S (2013) A study into the effect of POSS nanoparticles on cellulose acetate membranes. J Membr Sci 431:62–71. https://doi.org/10.1016/j.memsci.2012.12.025
Liu Y, Sun Y, Zeng F, Liu J, Ge J (2013) Effect of POSS nanofiller on structure, thermal and mechanical properties of PVDF matrix. J Nanopart Res 15:2116. https://doi.org/10.1007/s11051-013-2116-1
Toyodome H, Kaneko Y, Shikinaka K, Iyic N (2012) Preparation of carboxylate group-containing rod-like polysilsesquioxane with hexagonally stacked structure by sol–gel reaction of 2-cyanoethyltriethoxysilane. Polymer 53:6021–6026. https://doi.org/10.1016/j.polymer.2012.10.052
Kozuma T, Kaneko Y (2019) Preparation of carboxyl-functionalized polyhedral oligomeric silsesquioxane by a structural transformation reaction from soluble rod‐like polysilsesquioxane. J Polym Sci Part A: Polym Chem 57:2511–2518. https://doi.org/10.1002/pola.29519
Tsuru T (2008) Nano/subnano-tuning of porous ceramic membranes for molecular separation. J Sol-Gel Sci Technol 46:349–361. https://doi.org/10.1007/s10971-008-1712-5
Sato Y, Hayami R, Miyase Y, Ideno Y, Yamamoto K, Gunji T (2020) Preparation and properties of methyl- and cyclohexylsilsesquioxane oligomers as organic–inorganic fillers. J Sol-Gel Sci Technol 95:474–481. https://doi.org/10.1007/s10971-020-05291-2
Chan C-K, Peng S-L, Chu I-M, Ni S-C (2001) Effects of heat treatment on the properties of poly(methyl methacrylate)/silica hybrid materials prepared by sol–gel process. Polymer 42:4189–4196. https://doi.org/10.1016/S0032-3861(00)00817-X
Moriyama N, Nagasawa H, Kanezashi M, Ito K, Tsuru T (2018) Bis(triethoxysilyl)ethane (BTESE)-derived silica membranes: pore formation mechanism and gas permeation properties. J Sol-Gel Sci Technol 86:63–72. https://doi.org/10.1007/s10971-018-4618-x
Zhao L, Ho WSW (2014) Novel reverse osmosis membranes incorporated with a hydrophilic additive for seawater desalination. J Membr Sci 455:44–54. https://doi.org/10.1016/j.memsci.2013.12.066
Perera DHN, Nataraj SK, Thomson NM, Sepe S, Hüttner S, Steiner U, Qiblawey H, Sivaniah E (2014) Room-temperature development of thin film composite reverse osmosis membranes from cellulose acetate with antibacterial properties. J Membr Sci 453:212–220. https://doi.org/10.1016/j.memsci.2013.10.062
Yamamoto K, Ohshita J, Mizumo T, Kanezashi M, Tsuru T (2015) Preparation of hydroxyl group containing bridged organosilica membranes for water desalination. Sep Purif Technol 156:396–402. https://doi.org/10.1016/j.seppur.2015.10.028
Funding
This work was supported by JSPS KAKENHI [grant number JP12345678]. This research was supported by the Adaptable and Seamless Technology transfer Program through Target-driven R&D (A-STEP, Tryout JPMJTM20CG) from the Japan Science and Technology Agency (JST).
Author information
Authors and Affiliations
Contributions
Kazuki Yamamoto: Conceptualization, Writing—original draft. Yunosuke Amaike: Data curation. Miyuki Tani: Data curation. Ibuki Saito: Data curation. Tomoya Kozuma: Data curation. Yoshiro Kaneko: Conceptualization, Writing—reviewing and editing. Takahiro Gunji: Supervision, Writing—reviewing and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Yamamoto, K., Amaike, Y., Tani, M. et al. Bridged organosilica membranes incorporating carboxyl-functionalized cage silsesquioxanes for water desalination. J Sol-Gel Sci Technol 101, 315–322 (2022). https://doi.org/10.1007/s10971-021-05703-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-021-05703-x