Abstract
The influence of Sc3+ ion content on the microstructure, electrical, and gas-sensing properties of Ni0.5Co0.5ScxFe2–xO4 (x = 0.0, 0.05, 0.1 and 0.2) ferrites synthesized by a novel self-combustion method using polyvinyl alcohol as the colloidal medium, was studied. X-ray diffraction, X-ray photon spectroscopy (XPS), Brunauer–Emmett–Teller (BET) surface area, scanning electron microscopy (SEM), and energy-dispersive X-ray analysis (EDX) were employed to characterize the structure and morphology properties of these ferrites. The gas-sensing properties of hydrogen, methane, ethanol, methylene chloride, and benzene were investigated. The samples show p-type semiconducting properties for the studied gases within the temperature range of 100–380 °C. The results revealed that the partial substitution of Fe3+ by Sc3+ ions on the octahedral sites of the spinel structure of Ni0.5Co0.5Fe2O4 ferrite has a favorable effect on the sensing activity of this ferrite. The increase of the degree of Fe3+ ion substitution by Sc3+ ions up to x = 0.2 in the basic composition (Ni0.5Co0.5Fe2O4) results in the increase of the response and the decrease of the optimal operating temperature for all the studied gases. The sensor element Ni0.5Co0.5Sc0.2Fe1.8O4 (x = 0.2) has the best response to benzene (2.57) and to methylene chloride (2.10) at the operating temperature of 175 °C for a gas concentration of 500 ppm and a relative humidity of 50%.

Highlights
-
The Ni0.5Co0.5ScxFe2–xO4 (0 < x < 0.2) ferrites have been prepared by self-combustion method.
-
The porosity is amplified by a system with open pores distributed along the grain agglomerations.
-
The increase of substitution by Sc results in the increase of sensor response to the studied gases.
-
The substitution with Sc ions has the effect of decreasing the optimal operating temperature.
-
The Ni0.5Co0.5Sc0.2Fe1.8O4 sensors have the best response to benzene and methylene chloride gas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Spinel ferrites are the class of oxide semiconductor materials with remarkable electrical, magnetic, catalytic, and gas-sensing properties, which have been investigated and applied during the last few decades [1,2,3,4,5,6,7]. Ferrites with a spinel structure are materials with the composition AB2O4 (where A is a bivalent metal and B a trivalent metal), which have the crystalline structure of the mineral MgAl2O4 named spinel. In the case of spinel ferrites, the A ion can be any bivalent metal (Mg, Mn, Ni, Cu, etc.), and the B ion is Fe3+. The unit cell of spinel ferrites is composed of 32 oxygen atoms in a cubic close-packed arrangement distributed on tetrahedral (A) and octahedral sites (B). The chemical and structural properties of spinel ferrite nanocrystals are affected by their compositions and synthesis methods, and the corresponding electrical, magnetic, catalytic, and gas-sensing properties depend on cation substitutions [8,9,10].
The gas particles (atoms and/or molecules) interact with oxide semiconductor surfaces and thus influence the surface properties of compounds, in particular, their conductivity and surface potential. As compared with organic (phenanthrene and polybenzimidazole) and the elementary (Si, Ge, GaAs, and GaP) semiconductors, the oxide semiconductors can be successfully used as sensitive materials (resistive sensors) for detecting different gases: carbon monoxide and dioxide, hydrogen, alcohol, water vapors, ammonia, oxygen, nitrogen oxides, etc. Both n-type and p-type semiconductor oxides can be used as sensor elements [11, 12].
The used preparation method, composition, and morphology play an essential role for obtaining new gas-sensing materials. The spinel-type oxide semiconductor compounds have proved themselves to be suitable for detection applications of both reducing and oxidizing gases [12,13,14,15,16,17,18,19].
Kapse V.D. [17] has performed a study on some simple spinel ferrites (NiFe2O4, ZnFe2O4, MgFe2O4, ZnAl2O4, CoAl2O4, and MgAl2O4) synthesized by citrated sol–gel method, concerning their response (S) to different gases, such as hydrogen sulfide (H2S), ammonia (NH3), ethanol (C2H6O), and liquefied petroleum gas (LPG). He obtained the best response values (S = Rair/Rgas) for the spinel MgFe2O4 at three of the four studied gases, namely the response value is 4.8 for H2S, 12.4 for C2H6O, and 6.3 for LPG at an operating temperature of 325 °C and a concentration of 50 ppm. The CoAl2O4 spinel presents the best response value for NH3 (1.3) at an operating temperature of 150 °C.
Sutka et al. [18] have studied the Ni ferrite with Zn substitutes (Ni1–xZnxFe2O4) with respect to a series of structural, electrical, properties, and sensitivity to acetone (C3H6O), toluene (C7H8), propanol (C3H8O), and xylene (C8H10). The behavior of a p-type semiconductor demonstrated in the case of NiFeO4 ferrite modifies; namely, by the substitution with Zn ions, the ferrites show an n-type semiconductor behavior. The samples were synthesized by the sol–gel self-combustion method. In the case of the sample with x = 0, they obtained a response value (S = ∣Rair – Rgas∣/Rair) of 3.7 at acetone, 3.4 at propanol, 1.6 at xylene, and 1.1 at toluene, for a concentration of 500 ppm vapors in air at operating temperatures between 200 and 275 °C. For big values of Ni ion substitution by Zn ions (x = 0.7), they obtained a response value (S = ∣Rair – Rgas∣/Rgas) of 1.4 at acetone, 0.7 at propanol, 0.35 at xylene, and 0.2 at toluene.
Satyanarayana et al. [19] performed a study on a series of complex ferrites, among which is the Ni–Co ferrite (in various ratios) with Mn substitutions (Ni1−xCoxMnxFe2−xO4). The materials were synthesized by the hydrazine method and studied for gas-sensing behavior to reducing gases like liquefied petroleum gas, ethanol, carbon monoxide (CO), and methane (CH4). For Ni0.98Co0.02Mn0.02Fe1.98O4 ferrite, the authors obtain a response value (S = |Rair – Rgas|/Rair) of around 0.98 for LPG, 0.62 for CO, 0.48 for ethanol, and 0.11 for CH4 at a gas concentration of 1000 ppm and optimal operating temperature ranging between 210 and 230 °C.
In this work, we meant to improve the sensing properties of Ni0.5Co0.5Fe2O4 by partial substitution of Fe3+ ions by Sc3+ ions. High porosities and high surface area are preferred for gas sensor application. For these reasons, the researchers try new routes to prepare semiconductor oxide materials [11, 20, 21]. We have chosen the novel self-combustion method using polyvinyl alcohol as the colloidal medium [20, 22,23,24]. From our previous experiments concerning the realization of oxidic, spinel, and perovskite semiconducting materials, we found that this method could be used to obtain materials with properties favorable to some bulk-type sensors, such as gas sensors [11, 22, 23]. The rapid heating and cooling during self-combustion reaction produce nanopowder, from which samples with a high specific surface area and high effective porosity were obtained, which is beneficial for gas detection [11]. The composition, microstructure, electrical, and gas-sensing properties of hydrogen, methane, ethanol, methylene chloride, and benzene were investigated. The gas-sensing properties were determined by measuring the sensor element resistance in terms of various factors, such as operating temperature, type, and concentration of the test gas, and finally, the response time.
2 Experimental
2.1 Synthesis and characterization of materials
Nanopowders of Ni0.5Co0.5ScxFe2–xO4 ferrite with x = 0, 0.05, 0.10, and 0.20 (NCSF-0, NCSF-1, NCSF-2, and NCSF-3, respectively) were prepared by a novel self-combustion method using polyvinyl alcohol as fuel and as colloidal medium. The method included the following procedures: (1) dissolution of metal nitrates (stoichiometric amounts of analytical grade) in deionized water, (2) addition of polyvinyl alcohol solution, (3) addition of ammonia to increase the pH to about 8, (4) stirring at 80 °C, (5) drying the gel at 100 °C, and finally (6) self-combustion. The combusted powders were calcined at 500 °C for 30 min to eliminate any residual carbon and organic compounds.
The reactions for the basic composition (x = 0) can be schematized as follows:
The resulting powders were subjected to cold uniaxial pressing (400 MPa) in disk-shaped samples (17-mm diameter, 1.2-mm thick), followed by heat treatment in air for 240 min at 900 °C [22, 23].
X-ray diffraction (XRD) studies of the material were carried out at room temperature within the Bragg angle range 20° ≤ 2θ ≤ 70° at a scan speed of 2° min–1 by an X-ray diffractometer (PANALYTICAL X’PERT PRO MPD) using CuKα radiation (λ = 1.54059 Ǻ). Crystalline phases were identified by using the “Crystallographica” program. Unit cell parameters of crystalline phases were determined with XLAT-Cell Refinement program. The average crystallite size was evaluated based on XRD peak broadening using the Scherrer equation [25]
were λ is radiation wavelength, β is the half-width of the peak, and θ is the Bragg diffraction peak angle. The morphology and elemental chemical composition were analyzed by using a JEOL-200 CX scanning electron microscope (SEM), equipped with an energy-depressive X-ray spectrometer (Genesis, EDX). X-ray photoelectron spectroscopy (XPS) was used to distinguish the oxidation state of the cations present on the surfaces. Textural characteristics were investigated by Brunauer–Emmett–Teller (BET) analysis using a Quantachrome Nova 2200 Instruments.
2.2 Measurements on sensors
The d.c. electrical resistivity was determined using the two-probe method. High-purity silver paint electrodes were applied to either face of the heat-treated disk for ohmic contacts without a junction.
The sensor element was executed by deposing two comb-type silver electrodes on one face of the heat-treated disk, using the screen-printing method. For the gas response measurements, the sensor element was mounted on a heater and placed in a glass chamber endowed with an installation for temperature, humidity, and gas concentration control.
The gas-sensing properties were investigated at various operating temperatures from 100 to 380 °C. The experiments were performed with five test gases: hydrogen (H2), methane (CH4), ethanol (C2H6O), methylene chloride (CH2Cl2), and benzene (C6H6). The sensor response, S is defined as the ratio of resistance change in test gas ΔR = Ra–Rg to the value of resistance in air Ra, where Rg is the sensor resistance in the presence of the test gas [18, 19].
The gas concentrations were 100, 200, 400, 600, 800, and 1000 ppm, respectively, and the relative humidity (RH) was 50%.
3 Results and discussion
3.1 Structure and morphology
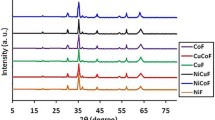
Figure 1 presents the X-ray diffractograms together with Miller indices for samples heat-treated in air at 900 °C for 240 min. From the analysis of X-ray diffractograms, it follows that the samples have a good quality of crystallinity and a cubic spinel-type structure (space group Fd3m) without secondary phases. The absence of secondary phases suggests a good solubility of scandium ions (under specified treatment conditions) even for a substitution x = 0.2 of the Fe3+ ions by Sc3+ ions (the NCSF-3 sample).
Table 1 presents the structural parameters, lattice constant (a), average crystallite size (DXRD), X-ray density (dx), specific surface area (SBET), and pore volume, of the samples heat-treated. One can see that the lattice constant slightly increases with increasing scandium concentration. The sample without scandium substitution (NCSF-0) has the lattice constant value in agreement with those reported by other authors referring to Ni–Co ferrites [26]. Lattice constant α increases slightly from 0.8365 nm, corresponding to the sample without scandium substitutions, to 0.8413 nm, corresponding to the sample with the biggest scandium substitution (NCSF-3), while the mean crystal size and X-ray density slightly decrease from 41.7 to 34.8 nm, and 5.43 to 5.33 g/cm3, respectively, when scandium content increases. A probable explanation for this slight decrease of mean crystal size and X-ray density, respectively, can be given by a structural disorder induced by the large Sc ions, which can lead to a delay in the crystallite growth. This can also explain, the progressive increase of the specific surface area, SBET, respectively increases the pore volumes from 26.5 to 32.3 m2/g and from 0.0052 to 0.0089 cm3/g, with the Sc content.
The analysis of the SEM micrographs realized on surfaces derived from cracking the heat-treated samples, revealed that the microstructure is affected by the amount of Sc3+ ions, that substitute the Fe3+ ions in the basic composition (Ni0.5Co0.5Fe2O4). Figure 2a, b shows the SEM micrographs for NCSF-0 (a) and NCSF-3 (b) samples. When scandium content increases, the average grain size (Dm) decreases from 560 nm corresponding to the sample NCSF-0 (Fig. 2a) to 175 nm corresponding to the sample NCSF-3 (Fig. 2b). Generally, the samples are characterized by a porous structure with clusters of particles, forming agglomerates. The Sc-substituted samples exhibit larger agglomerations and smaller-size grains in comparison with the undoped sample; probably, the presence of Sc ions inhibits the growth of the grains. The open-pore system distributed along the grain agglomerations arises during self-combustion reaction, through which a large amount of gas was eliminated. This phenomenon is common to all oxide compounds prepared through this method. These pores are necessary for sensor rapid response, because the gas adsorption rate is controlled by the rate of gas diffusion. These structures indicate that the investigated samples can easily exhibit adsorption and desorption of gases [11, 22, 24].
The crystallinity of the heat-treated samples was confirmed by the energy-dispersive X-ray spectra (EDX). Figure 3a, b presents the EDX spectrum of NCSF-0 (a) and NCSF-3 (b) samples. The obtained chemical elemental composition is typical for these ferrites (any foreign element is absent).
The XPS technique was used to identify the oxidation state of the cations present on the sample surfaces. Figure 4a, b shows the Fe 2p and Sc 2p XPS spectra, respectively, of ferrite with x = 0.2 Sc content (NCSF-3). The divalent Fe 2p3/2 peak at 709.5 eV and the trivalent Fe 2p3/2 peak at 711.2 eV are respectively associated with satellite peaks at 715.5 and 719.0 eV [27,28,29,30,31,32,33]. The registered Fe 2p3/2 peaks with binding energies (BE) from 711.2 to 713.0 eV, together with the associated satellites of 716.3 and 720.0 eV (Fig. 4a), confirm the prevalence of Fe3+ and a small fraction of Fe2+ present on the sample surface [27, 34, 35]. The observed BE shifts can be ascribed to the different surroundings of the Fe3+ ions in the A and B sites and to the Sc3+ ions (Fig. 4b) in the B (octahedral) site within ferrite structure.
3.2 Electrical and gas-sensing properties
Electrical resistivity measurements in air (ρa) at room temperature indicated very high values, of around 107 Ω cm for the sample without substitution by Sc ions (NCSF-0), and decrease slightly with the increase of Sc ion concentration, up to 106 Ω cm for the NCSF-3 sample. Because the gas sensitivity measurements were performed within the temperature interval 100–380 °C, we have investigated the temperature variation of the electrical resistance. The graph log ρa versus 1000/T for studied samples, given in Fig. 5, shows a decrease of ρa by three orders of magnitude in the above-mentioned temperature interval. Electrical resistivity ρa decreases with increasing temperature. The electrical conduction in Ni–Co ferrite is attributed to electron hopping between the two valence states of iron, Fe2+ and Fe3+ on the octahedral sites in the spinel lattice [36].
The activation energies (Ea) are obtained from the slope of each segment of the graphic, and their values decrease slightly with the concentration of Sc3+ ions, from 0.529 eV (NCSF-0) to 0.482 eV (NCSF-3). These values correspond to the semiconducting character of the material.
The grain may have a crystalline core and a coating with altered structure and composition. With increasing Sc content, the size of the grains decreases, the size of the crystalline core decreases, and the weight of the altered coating increases. Resistivity and activation energy are features of the surface of the granules, so they are influenced by the weight of the altered coating with the increase in Sc content.
Figure 6a–d shows the dependence of gas response vs. operating temperature for all the studied sensor elements, namely hydrogen, methane, ethanol, methylene chloride, and benzene at 500 ppm gas concentration and 50% relative humidity (RH). The samples show p-type semiconducting properties (the conductivity decreases in the presence of reducing gases because electrons released from the reaction would annihilate the holes) for the studied gases within the temperature range of 100–380 °C.
The increase of the degree of Fe3+ ion substitution by Sc3+ ions up to x = 0.2 on the octahedral sites of the spinel structure of Ni0.5Co0.5Fe2O4 ferrite, results in the increase of the response for the five studied gases, and the decrease of the optimum operating temperature (Fig. 6a–d).
In the case of the sample without Sc substitution, NCSF-0 (Fig. 6a), the response to all the five studied gases slightly increases with the operating temperature. The increase of the response gets more intense at operating temperature of around 200 °C for benzene and methylene chloride, and at a temperature higher than around 280 °C for hydrogen, methanol, and benzene; it reaches maximum values at an optimum operating temperature (Topt) of 350 °C, after which the response to gas decreases. In the case of this sample, the maximum response value is obtained at benzene (0.85), followed by methylene chloride (0.68), ethanol (0.58), hydrogen (0.52), and methane (0.26).
In the case of Sc-substituted samples, when the substitution degree increases, the response value increases, reaching a maximum for the NCSF-3 sample (Fig. 6d) for benzene (2.57), methylene chloride (2.1), ethanol (1.5), hydrogen (1.47), and methane (1.43).
The optimal operating temperature decreases from 350 to 320 °C for all the five test gases, in the case of the samples with moderate scandium substitutions NCFS-1 and NCSF-2 (Fig. 6b, c). A marked decrease of the optimum operating temperature (Top) is evident in the case of the NCSF-3 sample, with the highest substitution of Fe ions by Sc ions, for three of the five test gases, namely benzene, methylene chloride, and ethanol, while for hydrogen and methane, the optimal operating temperature remains at 320 °C. Figures 7 and 8 present as bar diagrams the values of the sensor response, respectively the values of the optimal operating temperature in terms of the value of substitution (x) of the Fe3+ ions by Sc3+ ions, for the five studied gases.
We should mention that all response measurements presented in this paper were collected at least 10–15 min after gas exposure, necessary to make the resistance steady. Presumably, this is the minimum time for the gas molecules to diffuse through the compressed ferrite powder. This time required to reach steady conditions does not change at different temperatures, suggesting that the gas diffusion into the porous sample is not critically dependent on the working temperature, but on the porosity within the ferrite.
The gas sensors that use oxidic compounds are able to detect different gases through the modification of their surface conductivity, due to the reaction of gases with the adsorbed oxygen [11, 37,38,39,40,41]. We can mention that with the increase of the operating temperature in clean air, the oxygen species adsorbed on Ni0.5Co0.5ScxFe2–xO4 surface suffer the following reactions:
where the subscripts gas, ads, and lattice mean the state of gas, adsorption, and lattice, respectively. The oxygen species capture electrons from the materials, leading to the increase of the hole concentration (h) [11, 22].
The Ni0.5Co0.5ScxFe2–xO4 samples studied by us are p-type semiconductor materials, and the charge carriers are obviously holes, whence the increase of sample conductivity through the increase of hole concentration. When the hydrogen (H2), methane (CH4), ethanol (C2H6O), methylene chloride (CH2Cl2), or benzene (C6H6) gas is introduced, a chemical reaction occurs between H2, CH4, C2H6O, CH2Cl2, and C6H6, respectively, and the adsorbed oxygen:
The electrons released from the reaction would annihilate the holes, leading to the increase of material resistivity. As each benzene molecule produces 15·n electrons (Eq. (18)), i.e., the highest number among the five gases, and the molar concentration was the same for all the five studied gases; the increase of the sensor element resistivity in the presence of benzene is the highest. This suggests that Ni0.5Co0.5ScxFe2–xO4 sensors are applicable to detect these gases, especially benzene vapors. Ethanol molecule produces 8·n electrons (Eq. (16)), the second in terms of electron weight, yet, it is on the third place concerning the response value, with methylene chloride on the second place. Even if its molecule produces only 3·n electrons (Eq. (17)), one should add the weight given by the influence of chlorine and the influence of the compounds from chlorine reactions with oxygen, chlorine dioxide, chlorine trioxide, dichlorine monoxide, etc. The substitution of Fe3+ ions by Sc3+ ions in Ni0.5Co0.5Fe2O4 intervenes directly, but in a different manner in the mechanism of the response to the five gases. For the studied gases, the response increases with increasing the concentration of Sc3+ (x) ions.
Figure 9 shows the changes in response for the sample with the highest substitution of Fe3+ ions by Sc3+ ions (NCSF-3), in terms of the stepwise increase of benzene and methylene chloride gas concentration. Measurements were made at an optimal operating temperature (175 °C) for various gas concentrations ranging between 100 and 1000 ppm. The sensor response significantly increases with increasing gas concentration up to 700 ppm, and has the tendency to saturate at gas concentrations above 800 ppm. The sensor element is able to detect 100 ppm benzene and methylene chloride vapors, respectively, with response values of 1.00 and 0.8, respectively, and 500 ppm with response values of 2.57 and 2.1, respectively.
Another important factor for every gas sensor consists of the response and recovery time, when the sensor is exposed to the gas environment and then removed from it. Figure 10 shows the curves of the NCSF-3 sensing element response to benzene and methylene chloride vapor, for a gas concentration of 500 ppm, at an optimal operating temperature (175 °C). The response time required for the response value to attain 90% of its maximum value is shorter, about 45 s for benzene and 40 s for methylene chloride. The time taken by the sensor element to come back once the test gas was removed is found to be longer, about 80 s for benzene and 75 s for methylene chloride. The response time considered by us as a short time for a bulk element sensor can be explained by material porosity, which is amplified by a system with open pores distributed along the grain agglomerations.
4 Conclusions
The nanocrystalline Ni0.5Co0.5ScxFe2–xO4 (x = 0, 0.05, 0.10, and 0.20) powder was prepared by self-combustion method, using polyvinyl alcohol as the colloidal medium, followed by heat treatment at 900 °C for 240 min. The compounds exhibit cubic symmetry (space group Fd3m) without secondary phases. With the increase of the amount of Sc ions that substitute Fe ions, the average grain size (Dm) decreases from 560 nm (x = 0) to 175 nm (x = 0.2). The samples are characterized by a porous structure with clusters of particles, forming agglomerates; open-pore systems appearing during self-combustion reaction are distributed along these agglomerates. The gas-sensing mechanism of the Ni0.5Co0.5ScxFe2–xO4 spinels has also been discused. The sensor elements show p-type semiconducting properties for all the studied gases (hydrogen, methane, ethanol, methylene chloride, and benzene) within the temperature range 100–380 °C. The increase of the degree of substitution of Fe3+ ions by Sc3+ ions up to x = 0.2 on the octahedral sites of the spinel structure of Ni0.5Co0.5Fe2O4 ferrite, leads to the increase of the response for all the studied gases and the decrease of the optimal operating temperature by 175 °C for benzene, methylene chloride, and ethanol, and by 30 °C for hydrogen and methane. The sensor element Ni0.5Co0.5Sc0.2Fe1.8O4 (x = 0.2) has the best response to benzene and methylene chloride. At a concentration of 500 ppm gas, at 50% of relative humidity and the operating temperature of 175 °C, the response to benzene is of 2.57 and to methylene chloride is of 2.10.
References
Velhal NB, Patil ND, Shelke AR, Deshpande NG, Puri VR (2015) Structural, dielectric and magnetic properties of nickel substituted cobalt ferrite nanoparticles: effect of nickel concentration. AIP Advances 5 097166:1–11. https://doi.org/10.1063/1.4931908
Šutkaa A, Grossab KA (2016) Spinel ferrite oxide semiconductor gas sensors. Sens Actuators B 222:95–105
Tanga X, Zhang B, Xiao C, Zhoub H, Wanga X, Hea D (2016) Carbon nanotube template synthesis of hierarchical NiCoO2 composite for non-enzyme glucose detection. Sens Actuators B 222:232–239
Tatarchuk T, Bououdina M, Vijaya JJ, John Kennedy L (2017) Spinel ferrite nanoparticles: synthesis, crystal structure, properties, and perspective applications. In: Fesenko O, Yatsenko L (eds) Nanophysics, Nanomaterials, Interface Studies, and Applications. NANO2016, vol 195. Springer Proceedings in Physics, Lviv, Ukraine, p 305–325 https://doi.org/10.1007/978-3-319-56422-7_22
Rezlescu N, Doroftei C, Rezlescu E, Popa PD (2008) Lithium ferrite for gas sensing applications. Sens Actuators B 133:420–425
Ibrahima I, Alia IO, Salamaa TM, Bahgatb AA, Mohamed MM (2016) Synthesis of magnetically recyclable spinel ferrite (MFe2O4, M=Zn, Co, Mn) nanocrystals engineered by sol gel-hydrothermal technology: high catalytic performances for nitroarenes reduction. Appl Catal B 181:389–402
Moussaoui HE, Mahfoud T, Habouti S, Maalam KE, Ali MB, Hamedoun M, Mounkachi O, Masrour R, Hlile EK, Benyoussef A (2016) Synthesis and magnetic properties of tin spinel ferrites doped manganese. J Magn Magn Mater 405:181–186
Xiangfeng C, Chenmou Z (2003) Sulfide-sensing characteristics of MFe2O4 (M=Zn, Cd, Mg and Cu) thick film prepared by co-precipitation method. Sens Actuators B 96:504–508
Kumar R, Singh RR, Barman PB (2014) Cobalt doped nickel zinc ferrite nanoparticles – XRD analyses an insight. Int J Sci & Eng Res 5:12–20
Mathew DS, Juang R (2007) An overview of the structure and magnetism of spinel ferrite nanoparticles and their synthesis in microemulsions. Chem Eng J 129:51–65
Doroftei C, Popa PD, Iacomi F, Leontie L (2014) The influence of Zn2+ ions on the microstructure, electrical and gas sensing properties of La0.8Pb0.2FeO3 perovskite. Sens Actuators B 191:239–245
Doroftei C, Prelipceanu OS, Carlescu A, Leontie L, Prelipceanu M (2018) Porous spinel-type oxide semiconductors for high-performance acetone sensors. In: 2018 International Conference on Development and Application Systems (DAS), IEEE, Suceava, 24–26 May 2018, p 110–114 https://doi.org/10.1109/DAAS.2018.8396081
Bangale SV, Patil DR, Bamane SR (2011) Nanostructured spinel ZnFe2O4 for the detection of chlorine gas. Sens Trans J 134:107–119
Reddy CVG, Manorama SV, Rao VJ (1999) Semiconducting gas sensor for chlorine based on inverse spinel nickel ferrite. Sens Actuators B 55:90–95
Comini E, Ferroni M, Guidi V, Fagila G, Martinelli G, Sberverglieri G (2002) Nanostructured mixed oxides compounds for gas sensing applications. Sens Actuators B 84:26–32
Niu X, Du W, Du W (2004) Preparation and gas sensing properties of ZnM2O4 (M=Fe, Co, Cr). Sens Actuators B 99:405–409
Kapse VD (2015) Preparation of nanocrystalline spinel-type oxide materials for gas sensing applications. Res J Chem Sci 5:7–12
Sutka A, Mezinskis G, Lusis A, Stingaciuc M (2012) Gas sensing properties of Zn-doped p-type nickel ferrite. Sens Actuators B 171–172:354–360
Satyanarayana L, Reddy KM, Manorama SV (2003) Synthesis of nanocrystalline Ni1−xCoxMnxFe2−xO4: a material for liquefied petroleum gas sensing. Sens Actuators B 89:62–67
Doroftei C, Popa PD, Iacomi F (2013) Selectivity between methanol and ethanol gas of La-Pb-Fe-O perovskite synthesized by novel method. Sens Actuators A 190:176–180
Thaweechai T, Wisitsoraat A, Laobuthee A, Koonsaeng N (2009) Ethanol sensing of La1-xSrxFeO3 (x=0, 0.1 and 0.3) prepared by metal organic complex decomposition. Kasetsart J (Nat. Sci.) 43:218–223
Doroftei C, Popa PD, Iacomi F (2012) Synthesis of nanocrystalline La-Pb-Fe-O perovskite and methanol-sensing characteristics. Sens Actuators B 161:977–981
Rezlescu N, Doroftei C, Rezlescu E, Popa PD (2008) Lithium ferrite for gas sensing applications. Sens Actuators B 133:420–425
Rezlescu N, Popa PD, Rezlescu E, Doroftei C (2008) Microstructure characteristics of some polycrystalline oxide compounds prepared by sol-gel-selfcombustion way for gas sensor applications. Rom J Phys 53:545–555
Cullity BD, Stock RS (2001) Elements of X-ray diffraction, 3rd edn. Prentice Hall, New Jersey
Akbarnejad RH, Daadmehr V, Rezakhani AT, Tehrani FS, Aghakhani F, Gholipour S (2013) J Supercond Nov Magn 26:429–435
Albuquerque AS, Tolentino MVC, Ardisson JC, Moura FCC, Mendonca R, Macedo WAA (2012) Nanostructured ferrites: structural analysis and catalytic activity. Ceram Int 38:2225–2231
Mittal VK, Chandramohan P, Bera S, Srinivasan MP, Velmurugan S, Narasimhan SV (2006) Cation distribution in NixMg1-xFe2O4 studied by XPS and Mössbauer spectroscopy. Solid State Commun 137:6–10
Yamashita T, Hayes P (2008) Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl Surf Sci 254:2441–2449
Vijayaraj M, Gopinath CS (2006) On the ”Active Spacer and Stabilizer” Role of Zn in Cu1-xZnxFe2O4 towards selective N-methylaniline from aniline: XPS and catalysis study. J Catal 241:83–95
Mathew T, Shiju NR, Bokade VV, Rao BS, Gopinath CS (2004) Selective catalytic synthesis of 2-ethyl phenol over Cu1-xCoxFe2O4—Kinetics, Catalysis and XPS aspects. Catal Lett 94:223–236
Munoz R, Martos M, Rotaru CM, Beltran H, Cordoncillo E, Escribano P (2006) Influence of the precursors on the formation and properties of the FexCr2-xO3 solid solution. J Eur Ceram Soc 26:1363–1370
Doroftei C, Leontie L (2017) Synthesis and characterization of some nanostructured composite oxides for low temperature catalytic combustion of dilute propane. RSC Adv 7:27863–27871
McIntyre NS, Zetaruk DG (1977) X-ray photoelectron spectroscopic studies of iron oxides. Anal Chem 49:1521–1529
Leontie L, Doroftei C (2017) Nanostructured spinel ferrites for catalytic combustion of gasoline vapors. Catal Lett 147:2542–2548
Smit J, Wijin HPJ (1961) Les ferrites, Dunot, Paris
Liu X, Cheng B, Qin H, Song P, Huang S, Zhang R, Hu J, Jiang M (2007) Preparation, electrical and gas-sensing properties of perovskite-type La1−xMgxFeO3 semiconductor materials. J Phys Chem Solids 68:511–515
Zhang L, Qin HW, Song P, Hu JF, Jiang MH (2006) Electric properties and acetone-sensing characteristics of La1−xPbxFeO3 perovskite system. Mater Chem Phys 98:358–362
Doroftei C (2016) Formaldehyde sensitive Zn-doped LPFO thin films obtained by rf sputtering. Sens Actuators B 231:793–799
Song P, Hu J, Qin H, Zhang L (2005) H2-sensing characteristics of nanocrystalline La0.8Pb0.2FeO3 prepared by sol–gel method. J Sol–Gel Sci Technol 35:65–68
Huang S, Qin H, Song P, Liu X, Li L, Zhang R, Hu J, Jiang M (2007) The formaldehyde sensitivity of LaFe1−xZnxO3-based gas sensor. J Mater Sci 42:9973–9977
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Doroftei, C., Leontie, L. The influence of Sc3+ ions on the microstructure, electrical, and gas-sensing properties of Ni–Co–Sc ferrite. J Sol-Gel Sci Technol 91, 654–663 (2019). https://doi.org/10.1007/s10971-019-05069-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-019-05069-1