Abstract
Nanocrystalline NiLaFe2O4 exhibit unique properties, which make them promising candidates for an inclusive range of applications such as actuators, magnetic resonance imaging, including gas sensing element due to its inverse spinel structure. It is requisite to revamp its ionicity, phase and magnetic properties. A primal approach has been chosen to fabricate NiLaFe2O4nanocrystals by co-precipitation technique at different calcination temperatures (300 °C, 400 °C, 500 °C, 600 °C) phase, and ionicity as well as magnetic properties. Changes in the structural characteristics of as-synthesized samples have been found by the inclusion of rare-earth elements in X-ray diffraction studies. Fourier transform infrared spectral studies embrace two absorption bands peaked at 400 and 500 cm−1representing the octahedral and tetrahedral sites. The transmission electron microscopy analysis depicts the tailored morphology of as-synthesized nanocrystal. The magnetization was determined by vibrating sample magnetometer and found that Hc increases with decrease in Ms and magnetostriction coefficient. These results can be partially described by the frailer nature of La3+–Fe3+ions which are equated to Fe3+–Fe2+ interaction. A model for inverse spinel ferrite has been used which refers as O2p itinerant electron model. The magnetization and the cation distributions of the La doped inverse spinel ferrites were elucidated using this model. The sensor designates with high selectivity, repeatability and fast transition at room temperature (305 K) towards ammonia gas in particular when related to ethanol, acetone and toluene. Low deposition cost makes it competent for developing a cost-effective ammonia sensor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Environmental changes have led to an incongruent effect and disquietude on the environment owing to ozone pollutants and proliferated use of fossil fuels. The earth’s global surface temperature in 2018 has increased rapidly by 0.83 °C (warmer than 1951–1980 statistics) in the recent past 5 years and was recorded as the fourth warmest year by National Aeronautics and Space Administration (NASA) and the US National Oceanic and Atmospheric Administration (NOAA) [1]. The unusual and unprecedented increase in earth’s global surface temperature is due to global warming, leading to a substantial hike in earth’s global average temperature which is referred due to El-Nino effect [2]. The barriers have been effectively scrutinized by implementing the Paris agreement [3] thereby minimising deterioration of environmental degradation in terms of ecologically balanced, safe and healthy environment and it has been the contour of deliberations globally till date.
Air pollution, a menace for human beings and primate to various respiratory diseases such as asthma, chronic bronchitis, damage of cells in the respiratory system, primarily due to urbanisation, industrialisation and unprecedented diffusion of toxics and industrial waste to soil matrix and gas atmosphere. The automobile industries are insistent to use gas sensors for monitoring atmospheric pollution. Work on gas sensors have become an attractive goal globally as they are pertinent to monitor traffic pollutants and industries. This enhances research in gas sensing properties of ferrites to effectuate at room temperatures with stability, low cost, virtuous and favourable sensing properties to sense ammonia, ethanol, toluene and acetone as a reference mark to freedom from threats of pollution [4]. The role of calcination in nickel lanthanum ferrites at room temperature have been explored further in inverse spinel ferrites, which have lucrative applications in high-density recording devices, magnetic refrigerators, catalysts, gas sensors and medical diagnostics ascribed to its excellent electrical and magnetic properties. The magnetic behaviour of NiLaFe2O4 has drawn significant interest and has been subject of intensive studies in the recent years, influenced with nanoscopic size enhancement of single magnetic domain and disparity of super para-magnetization in the typical multi domain structure of bulk magnetic material [5]. This type of ferrite gas sensors can be used in automobile industries to study the air pollution. The super paramagnetic behaviour of magnetic nanoparticle unveils higher saturation magnetization and low coercivity with inherent utilization in magnetic resonance imaging, information storage device and gas sensors [6]. Consequently, it is fascinating to investigate on the gas-sensing properties of nickel lanthanum ferrites especially, the microstructural properties that lead to an increase in gas sensing efficiency and magnetic properties. Though there are several goal oriented attractive techniques for synthesizing nanosized magnetic inverse spinel ferrite nanoparticles like sol–gel [7], hydrothermal [8] combustion [9], mechano-chemical [10], microemulsion method [11], co-precipitation becomes the appropriate technique that is vital for maintaining highest degree of homogeneity for the preparation of NiLaFe2O4nanoparticles which yields ultrafine particles that are functionally influenced by composition and microstructure matrix [12].
In this investigation, gas sensing properties strongly depend on stability, selectivity, response, reproducibility and recovery time of gases such as ammonia, toluene, ethanol and acetone widely rely on the surface morphology, grain size, porosity, thickness, etc. The distribution of cations between two sub-lattices (tetrahedral and octahedral sites) is ideal for electric and magnetic properties [13]. Hence, the O2p itinerant electron model and the quantum mechanical potential barrier methods were used to investigate the cation distribution in several series of inverse spinel ferrites.
2 Experimental techniques
2.1 Synthesis
In this analysis, analytical graded Merck reagents, viz., ferric nitrate Fe(NO3)3·9H2O, nickel nitrate Ni(NO3)3·6H2O, sodium hydroxide NaOH, lanthanum nitrate La(NO3)3·3H2O were used to synthesize the candidate material by co-precipitation technique. The mixed solution of Ni(NO3)3·6H2O, Fe(NO3)3·9H2O and La(NO3)3·3H2O were prepared and kept at 60 °C and the NaOH was added carefully drop by drop to the boiling solution in order to convert hydroxide to ferrites. Then the solution was stirred at a constant velocity and maintained at 80 °C for an optimum period of 1 h. The fine particles thus obtained at this stage were centrifuged at 8000 rpm twice with distilled water and once with ethanol to make it free from nitrate ions. The obtained mixer was dried in an oven at 75 °C for 24 h and subsequently calcinated at 300 °C for 3 h in muffle furnace to harvest the nanocomposite of nickel lanthanum ferrite nanoparticles. The similar procedure is followed for different calcination temperatures (400 °C, 500 °C, 600 °C).
2.2 Characterization
The structural properties of the as-synthesised material were analysed by X-ray diffraction (XRD) using a BRUKER D8 advance X-ray powder diffractometer with CuKα1 radiation (λ = 1.5406 Å) in order to find the crystallite size. The functional dependence of surface morphology and inter-planar spacing of NiLaFe2O4were analysed by a transmission electron microscope (TEM) (model JEM 2100). The Fourier transform infrared spectra (FTIR) was depicted from Perkin-Elmer spectrum system in the range of 400–4000 cm−1. The magnetic behaviour of as-synthesised nanocomposite was investigated using Lakeshore VSM 7410S vibrating sample magnetometer.
3 Results and discussion
3.1 Structural analysis
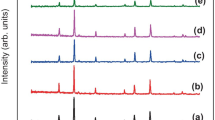
The X-ray diffraction patterns of rare earth (La3+) doped nickel ferrite nanocrystal synthesized via co-precipitation technique at different calcination temperatures are shown in Fig. 1. In phase analysis, the six pertinent peaks (220) (311) (400) (422) (511) and (440) matched well with the standard JCPDS file number (74-2081), exhibiting inverse spinel structure of fd-3m space group (Fig. 2). The X-ray diffractogram reveals the poly-crystalline nature of the nanocrystals. As the calcination temperature increases, the width of the peak and the crystallite size was progressively decreased, which specifies that the nanocrystals are constituted by finer particles. The broadening of peaks confirms that by doping of lanthanum in nickel ferrite, the surface to volume ratio increases for different calcination temperature [14] (Fig. 3).
The mean crystallite size was calculated from the supreme typical (311) peak by Scherrer’s equation:
where D is the mean crystallite size (nm), λ is the X-ray wavelength, k is the crystallite-shape factor, β is the full width at half maximumand θ is the Bragg angle. The mean crystallite size and intensity of the diffraction pattern decrease with increase of the calcination temperature as shown in Table 1. This demonstrates that the complete crystallization of NiLaFe2O4 relies on calcination temperatures. Hence, the gradual decrease in the crystallite size with the calcination temperatures shows the formation of larger particles and it is attributed to grain growth of the particles in nanoregion.
The lattice parameters of NiLaFe2O4 nanocrystal were calculated by the relation:
where d is the inter-planar distance, a is the lattice constant and (hkl) represents Miller indices.
The broadening βr is,
where βi is the instrumental broadening and βo is the observed broadening.
The X-ray density was calculated by:
where dx is the X-ray density, M is mass, N is the Avogadro number and a3 is the volume of the cubic unit cell.
The porosity of the material was determined using:
where dx the X-ray density and d is the apparent density.
The dislocation density was found using the below equation:
where m is the mass, h is the thickness and r is the radius. The calculated parameters values are listed in Table 1.
3.2 Infrared absorption spectra
The Fourier transform infrared Raman spectroscopy (FTIR) absorption of NiLaFe2O4depicts well-defined two vibrational peaks in the region of 400–600 cm−1. The bands in tetrahedral site υA (MA–O) and octahedral site υB (MB–O) is due to the stretching vibration modes of metal complex. The position of two bands υB and υt are related to the intrinsic vibration of the inverse cubic spinel structure [15]. The higher frequency absorption υt in the range of 600 cm−1 and the lower frequency absorption υo in the range of 400 cm−1 corresponds to the two interstitial complexes. The position of the A site in the FTIR spectra is at a higher region as compared to that of the B site due to the difference in Fe3+–O2 because of introducing lanthanum in nickel ferrite. In tetrahedral lattice Fe–O has shorter bond length and the energy is given to vibrate the bond in the position of octahedral lattice.
The stretching band is conversely corresponding to the reduced mass by the Eqs. (7) and (8):
where m1 and m2 are the molecular weight of the atoms, c is the speed of light and k is the force constant. Since, the molar mass of La3+ is higher than Fe3+ and Ni3+ ions, doping of La3+ ions leads to weakening and decrease of metal oxygen bond in both υA and υB sites in the inverse spinel structure shown in Fig. 4.
Furthermore, to equipoise the strain energy, doping of La3+ ions in octahedral sites induce the relocation of an equal amount of Ni2+ions from B to A sites, thus an equal amount of Fe3+ ions migrate from A to B sites [16]. The ionic radii of Ni2+ ions are higher than Fe3+ ions and so increasing the mean ionic radii of Ni2+ ions in A-sites (rA). Similarly, the number of La3+ ions increase in B-sites (rB). A small shift occurs towards the lower wavenumber due to the change in bond length of metal oxygen and of fundamental stretching band wavenumber of A and B sites. The strengthening of inter ionic bond was found via the force constant by doping of La3+ in nickel ferrite for different calcination temperatures [17]. Thus, the values of force constant (kt) and (k0) are maximum for NiLaFe2O4 due to higher molecular weight. The force constant k0 and kt were calculated by following equations:
where k0 and kt are force constant of A and B sites, υA and υB represents the wave number absorption in octahedral and tetrahedral sites, M is the molecular weight and it was determined that both the bond length and force constant decreased linearly for different calcination temperature of NiLaFe2O4shown in Table 2.
The bond length was given by the equations:
where Ro is the radius of oxygen ions and u is the oxygen positional parameter.
3.3 Transmission electron microscopy (TEM)
The as-synthesised NiLaFe2O4 nanoparticles for various calcination temperatures were tailored by co-precipitation technique and the resultant morphology is depicted in TEM micrograph; the formation of grain boundaries and nano crystallite size was determined by increasing the calcination temperatures up to 600 °C [18, 19], hence the surface morphology and particle pattern of as-synthesized nanocomposite are seen in Fig. 5. The crystallite size increases for different calcination and the agglomeration is understood due to magnetic property of NiLaFe2O4, henceforth some degree of agglomeration that appears at higher calcination temperatures which was controlled during the synthesis by adapting the nucleation growth rates [20].
3.4 Vibrating sample magnetometer (VSM)
The various parameters such as saturation magnetization, coercivity, retentivity and exchange interaction of the NiLaFe2O4 nanocomposites for different calcination temperatures are shown in Fig. 6.
3.4.1 Magnetic properties
The magnetic properties of the as-synthesised nanocomposites begin from the quantum coupling at nuclear level, also it includes a coupling between the electron spin (S–S coupling) and orbital angular momentum (L–S coupling) [21]. By doping lanthanum in nickel ferrite have the propensity to possess a single magnetic domain. Therefore, these as-synthesised nanocomposites afford an exceptional probability to investigate the relationship between the coupling and the magnetic behaviour [22]. Hysteresis loop shows a result of change in distribution of cations and particle pattern.
3.4.2 Saturation magnetization
The saturation magnetisation shows monotonous increase with calcination temperatures. This can be due to the presence of paramagnetic ions at room temperature, which decreases the negative exchange interactions between multi-domains to single domain transition behaviour in NiLaFe2O4[23]. In the multi-domain, the magnetisation changes by domain wall and the coercivity decreases as the crystallite size decreases. In this study, it was observed that there is an increase in saturation magnetization with respect to decrease in particle sizes. In our attempt, NiLaFe2O4was calcinated at higher temperatures consist of single-domain nanoparticles. Thus, the increase in saturation magnetisation with the decrease of particle size could be attributed with surface effects that would be the consequence of finite-size scaling about nanocrystallites, hence it prompts a non-collinearity of magnetic moment with respect to their surface [24]. As the calcination temperature is beneath 600 °C, the saturation magnetisation indicates lower values than that of bulk of the as-synthesised material.
3.4.3 Exchange interactions
Intrinsic magnetic properties rely upon the exchange interactions, and the exchange interaction strongly depends upon the bond angles and inter-ionic length. In our attempt, due to the particle size there is an exchange in length and bond angle [25]. The high value of saturation magnetization of NiLaFe2O4nanoparticles has been obtained from co-precipitation route shown in Table 3, which explains the general process in the bond angles to similar sites like A–A, B–B and also increases the A–B sites.
According to the quantum mechanical potential barrier model and O2P itinerant electron model, the estimation of the ion distribution in inverse spinel ferrites involves four important aspects, to study the magnetic moments which affect the cation distribution [26].
First, O2− molecules loses an electron due to the ionisation energy of the electron. The cation ionization energy is very significant due to the distance between cation and anion pair. The height and width of the potential barrier depend on the ionization energy and located between cation and anion pair of inverse spinel ferrites. The probability of these final ionized electrons was transmitted through the potential barrier is represented as,
where TC and TD signify probability of final ionized cation moving towards the anions through the square potential barrier. VD and VC are the width and height of the potential barrier. rD and rC are the distance from the cations to anions and c is a barrier shape modifying the constant which is interrelated to the potential barrier depart from the shapes of the other two barriers.
Second, from the ionization energies of the electrons, we consider the Pauli repulsion energy of the electron among neighbouring ions. In addition, this can be considered as the effective ionic radii by increasing the temperature of as-synthesised material, the lattice constant increases and the smaller ions would relocate to the sites with smaller available space in the lattice. For instance the accessible volume of the A site is lower than those of the B sites in the inverse spinel ferrites.
Third, the ionicity of a compound has an extensive impact. Since, the O2p itinerant electron model is characterised by properties like ionicity and the fraction of ionic bonds. In the sub-lattice, cation can act as an intermediary, were the O2p electron has a constant spin direction which can move from an O2− anion to the O2p hole of an adjacent O1−. The two different sublattices of A and B has an itinerant electrons for the opposite spin directions of two O2p in the outer orbit of an O2−anion. The sublattices are constrained by Hunds rule and an itinerant electron which has a constant spin direction, the direction of magnetic moments of cations with the 3d electron number. The magnetic moment of Ni3+, La3+, Fe3+ ions present either in the A or B sub lattice.
Fourth, some of the bivalent ions enter the A-sites from the B-sites by jumping over an equivalent potential barrier, since the ions tend to obtain a balance in charge density, which ascends from the Pauli repulsion energy, magnetic energy and ionization energies of the electrons. Hence cations at B-sites have six adjacent oxygen ions, whereas the cations at A-sites have four adjacent oxygen ions. Thus, VBA arise from the Pauli repulsion energy of the electron cloud as well as from the magnetic ordered energy.
If we generalize the composition of NiLaFe2O4according to Tang et al. estimation method [27]. The composition of NiLaFe2O4and valence of various ions was represented by the following equations:
The chemical formula of the NiLaFe2O4 can be written as:
Subject to the constraints arising from the valence ion and the compositions of cations is given by,
From Eq. (16),
where N3is the number of trivalent cations and \(f_{{{\text{Ni}}}} ,f_{{{\text{la}}}} ,f_{{{\text{Fe}}}}\) represents the ionicities. From the Eq. (16) we can obtain,
where RA1, RA2, RA4, RA5 and RA6 signify the ratios of La2+, Ni2+, and Fe2+ ions in A-sites, RB1 and RB2 shows the probability of the La3+ and Ni3+ ions in B-sites.
From Eqs. (17) (20) (21) (22) (23) and (24),
and from Eqs. (18, 25 and 26),
According to the quantum mechanical potential barrier method for estimating the cation distribution with inverse spinel ferrites, RA1, RA2, RA4 and RA5 at the A-sites and RB1, RB2at the B-sites can be derived as,
where \(V_{{{\text{BA}}}} \left( {{\text{La}}^{{2 + }} } \right),V_{{{\text{BA}}}} \left( {{\text{Ni}}^{{2 + }} } \right)\quad {\text{and}}\quad V_{{{\text{BA}}}} \left( {{\text{Fe}}^{{2 + }} } \right)\) are known to be the heights of the potential barriers which is transmitted through La2+, Ni2+, and Fe2+ ions as they move from B to A-sites shown in Table 4.
According to the O2p itinerant-electron model, the magnetic moments of the La3+, Ni2+, and Ni3+ ions are antiparallel to La2+, Fe3+ and Fe2+ cations in the identical sub lattice of an inverse spinel ferrite [19]. Hence, we can calculate the average magnetic moment per formula unit of a sample from Eq. (16),
where μC is the calculated magnetic moment and μBT the magnetic moments of the A and B sub-lattices.
The Fe content at the B sites is clearly greater than the other cation contents for as-synthesised material of the two series, therefore the direction of the magnetic moments is same as that of the Fe cations at the B site [28]. The La doping at B site for different calcination temperatures leads to the most distinct change in the cation distribution among the nickel ferrite ion; thus, resulting in an increased magnetic moments of the samples with an increase in the calcination temperature [29]. This results in decrease in magnetic moments of the as-synthesised nanocomposite for different calcination temperatures. Ni cation content in the A sites decreasing continuously with increasing calcination are shown in Fig. 7. Therefore, a quantum mechanical estimating ionicity is proved to be suitable for IV compounds. Employing this method, ionicities of inverse spinel ferrites were estimated. On the basis of this, the ion distributions at A site and B sites of NiLaFe2O4 ((A)[B]2O4) ferrites was calculated using ionicities values [30].
4 Ammonia vapour sensing studies
A sealed airtight chamber was used for sensing characterization with controlled gas inlet and outlet valves as shown in Fig. 9. The doctor blade method was used to prepare the thin layer of the nanostructured films. In a typical process, the colloidal paste was prepared using the following steps 0.1 g of NiLaFe2O4nanoparticles was ground with help of appropriate amounts of distilled water and acetylacetone for 10 min to form a viscous paste. The viscous paste was slowly added with 0.3 ml of distilled water until desirable viscosity was attained Finally, 20 μl of surfactant (Triton X-100) was slowly added with grinding for 10 min. The resulting NiLaFe2O4 paste is uniformly dispersed on the conducting substrate using the doctor blade method (Fig. 8). After coating, the films were dried in air atmosphere at room temperature. Then the films were heat treated at 200 °C for 30 min.
The coated thin film was placed in sample stage chamber with silver paste on the edges of thin film to assure Ohmic contact between probe and sample is shown in Fig. 9. The working temperature of the sensor was maintained at room temperature of 35 °C. The variation in resistance and current was analysed using Keithley 2450 source meter for time interval of 0.5 s with source voltage. The sensing instrument was monitored using a computer running GUI developed Lab VIEW.
The base resistance was taken from surrounding air and as it is stabilized, the gas was allowed to enter into the chamber using gas inlet valve. The sensitivity was analysed by the change in resistance using the relation,
where Ra and Rg are the resistance in air and in gas.
The concentration of the input gas was calculated using relation,
where δ is the density of testing gas (g ml−1), V is the volume of the injected test gas (μl), R is the universal gas constant (8.415 J mol−1 K−1), T is the sensor temperature (K), M is the molecular weight of the testing injected gas (g mol−1), Pb is the chamber pressure and Vb is the volume of the chamber (litres).
4.1 Response and recovery studies
The transient resistance response of the NiLaFe2O4 for different calcination temperatures thin film sensor was tested under ammonia vapour concentration ranging from 10 to 100 ppm at room temperature ∼ 35 °C is shown in Fig. 10. The thin film was exposed to a dry air atmosphere which exhibited higher resistance due to the oxidation reaction between surface electrons and oxygen molecules at the beginning of the sensing element experiment. A sharp drop in the resistance of the film was observed due to the instigation of ammonia vapour into the testing chamber [31]. Moreover, an increase in the resistance of the film was observed when the ammonia vapour was removed from the chamber through vacuum pump, leading to a baseline resistance. A fast response was observed at higher concentration due to the interaction of more ammonia molecules with adsorbed oxygen ions. Figure 11 depicts the very dominant response at 600 °C compared to other calcination temperatures [32, 33]. However, NiLaFe2O4 at 300–500 °C was found to be saturated above 25 ppm, but an increased response was observed at 600 °C thin film, even up to 100 ppm. Hence, the NiLaFe2O4 at 600 °C sensing element can be used to detect wide range of concentration of ammonia with better selectivity.
Figure 11 shows the time-dependent response and recovery time curves for 50 ppm of vapour, which clearly demonstrates the different steps involved in the sensing. At high concentrations, more ammonia molecules easily interact with adsorbed oxygen ions, leading to a fast response. The observed slow recovery may be due to room temperature operation [34]. As the concentration increases from 10 to 100 ppm, the response time was found to decrease from 181 to 198 s, while the recovery time increases from 64 to 75 s. However, the observed fast response–recovery times (in the order of seconds) is due to smaller grain size compared to the ammonia sensing properties of different calcination temperature at 300 °C, 400 °C, 500 °C and 600 °C nanostructured NiLaFe2O4thin film sensor developed in the present study. Mabrook and Hawkins [35] investigated the room temperature ammonia sensing behaviour of NiLaFe2O4 thin films, which results in a less sensing response, minimum detection limit of 100 ppm and long response recovery time.
4.2 Sensing response to different reducing vapours and selectivity
The sensing response of NiLaFe2O4at different calcination temperature towards different reducing vapours such as ammonia, ethanol, acetone and toluene at a fixed concentration of 50 ppm with error bar as shown in Fig. 12. The sensing response towards ammonia is higher than any other reducing vapours as observed. The estimated magnitude of the sensing response is 662% for 50 ppm of ammonia, and the response towards the other vapours is not greater than half of the magnitude for ammonia [36, 37]. The 50 ppm of ammonia vapour under dynamic cycling, the corresponding resistance decreased rapidly towards its equilibrium value. As the ammonia vapour was discharged from the chamber through vacuum pump, the resistance increased rapidly to the baseline value, indicating the good reproducibility and reversibility of the thin film response [38, 39]. The sensing experiments were repeated three times and statistical parameters such as the mean, median, standard deviation (SD) and co-efficient of variation (CV) for resistance (Rg), response (S) and response and recovery time (τ) [40] were estimated and is shown in Table 5.
5 Conclusion
The investigation was focussed on the La3+ substitution on NiLaFe2O4 by varying calcination temperatures.
-
1.
The crystallite size has a stronger effect on the magnetic properties than the degree of crystallinity and the cation distribution, respectively.
-
2.
The magnetization Ms increases with respect to increase in calcination temperatures. These magnetic properties are explained by the ferromagnetic nature of the ferrite.
-
3.
The ferromagnetic phase of A-sub lattice and paramagnetic phase of B-sub lattice start to appear with doping of lanthanum in nickel ferrite and found that increases with increasing calcination temperatures.
-
4.
The magnetization of the Ni3+ cations are antiparallel to the Fe3+ cations in the equal sub lattice of the as-synthesised NiLaFe2O4and it specifies that quantum mechanical method is vibrant for assessing the ion distribution.
-
5.
The ammonia sensing characteristics of NiLaFe2O4at different calcination temperature were evaluated and the results indicate a good sensing response, fast response time, short recovery time, and long term stability towards ammonia than other vapours, favorable reproducibility and low temperature operation. Thus the \(\mathrm{N}\mathrm{i}\mathrm{L}\mathrm{a}{\mathrm{F}\mathrm{e}}_{2}{\mathrm{O}}_{4}\) calcinated at 600 °C could be of great interest in the fabrication of ammonia analysers compared to that of other calcination temperatures.
References
J. Charlotte, The last five years have been Earth’s warmest since records began. (MIT Technologly Review, 2019), https://www.technologyreview.com/f/612908/the-last-five-years-have-been-earths-warmest-since-records-began/. Accessed 07 Feb 2019
C. Clark, G.A. Nnaji, W. Huang, J. Coast. Res. 68, 113–120 (2014)
C. Streck, P. Keenlyside, M. von Unger, J. Eur. Environ. Plan. Law 13, 3–29 (2016)
J. Kennedy, G.V. Williams, P.P. Murmu, B.J. Ruck, Phys. Rev. B 88, 1–5 (2013)
A.K. Nikumbh, R.A. Pawar, D.V. Nighot, G.S. Gugale, M.D. Sangale, M.B. Khanvilkar, A.V. Nagawade, J. Magn. Magn. Mater. 355, 201–209 (2014)
G.S. Rao, C.N.R. Rao, J.R. Ferraro, Appl. Spectrosc. 24, 436–445 (1970)
N.T. Tu, P.N. Hai, L.D. Anh, M. Tanaka, Phys. Rev. B 92, 1–14 (2015)
J.B. Silva, W. De Brito, N.D. Mohallem, Mater. Sci. Eng. B 112, 182–187 (2004)
P.A. Vinosha, K. Raja, A. Christina Fernandez, S. Krishnan, J. Das, Optik 127, 9917–9925 (2016)
X. Liu, Y. Sasaki, J.K. Furdyna, Phys. Rev. B 67, 1–9 (2003)
H. Wang, Y. Liu, M. Li, H. Huang, H.M. Xu, R.J. Hong, H. Shen, Optoelectron. Adv. Mater. Rapid Commun. 4, 1166–1169 (2010)
X. Meng, H. Li, J. Chen, L. Mei, K. Wang, X. Li, J. Magn. Magn. Mater. 321, 1155–1158 (2009)
H. She, Y. Chen, X. Chen, K. Zhang, Z. Wang, D.L. Peng, J. Mater. Chem. 22, 2757–2765 (2012)
Z. Karimi, Y. Mohammadifar, H. Shokrollahi, S.K. Asl, G. Yousefi, L. Karimi, J. Magn. Magn. Mater. 361, 150–156 (2014)
L. Avazpour, H. Shokrollahi, M.R. Toroghinejad, M.Z. Khajeh, J. Alloy Compd. 662, 441–447 (2016)
N. Bouhadouza, A. Rais, S. Kaoua, M. Moreau, K. Taibi, A. Addou, Ceram. Int. 41, 11687–11692 (2015)
J. Tauc, A. Menth, J. Non-Cryst. Solids 8, 569–585 (1972)
P.A. Vinosha, L.A. Mely, J.E. Jeronsia, S. Krishnan, J. Das, Optik 134, 99–108 (2017)
P. Kumar, S.K. Sharma, M. Knobel, J. Chand, M. Singh, J. Electroceram. 27, 51 (2011)
J. Jiang, Y.M. Yang, Mater. Lett. 61, 4276–4279 (2007)
S. Briceño, J. Suarez, G. Gonzalez, Mater. Sci. Eng. C 78, 842–846 (2017)
S. Deepapriya, P.A. Vinosha, J.D. Rodney, M. Jose, S. Krishnan, J.E. Jose, S.J. Das, Vacuum 161, 5–13 (2019)
K.K. Kefeni, T.A. Msagati, B.B. Mamba, Mater. Sci. Eng. B 215, 37–55 (2017)
S.R. Naik, A.V. Salker, S.M. Yusuf, S.S. Meena, J. Alloy Compd. 566, 54–61 (2013)
R.D.K. Misra, S. Gubbala, A. Kale, W.F. Egelhoff Jr., Mater. Sci. Eng. B 111, 164–174 (2004)
Y.N. Du, J. Xu, Z.Z. Li, G.D. Tang, J.J. Qian, M.Y. Chen, W.H. Qi, RSC Adv. 8, 302–310 (2018)
L.L. Ding, L.C. Xue, Z.Z. Li, S.Q. Li, G.D. Tang, W.H. Qi, L.Q. Wu, X.S. Ge, AIP Adv. 6, 1–23 (2016)
Q.J. Han, D.H. Ji, G.D. Tang, Z.Z. Li, X. Hou, W.H. Qi, S.R. Liu, R.R. Bian, J. Magn. Magn. Mater. 324, 1975–1981 (2012)
F. Huixia, C. Baiyi, Z. Deyi, Z. Jianqiang, T. Lin, J. Magn. Magn. Mater. 356, 68–72 (2014)
R.K. Sonker, S.R. Sabhajeet, S. Singh, B.C. Yadav, Mater. Lett. 152, 189–191 (2015)
I. Sandu, L. Presmanes, P. Alphonse, P. Tailhades, Thin Solid Films 495, 130–133 (2006)
S. Parthasarathy, V. Nandhini, B.G. Jeyaprakash, J. Colloid Interface Sci. 482, 81–88 (2016)
A.B. Gadkari, T.J. Shinde, P.N. Vasambekar, J. Alloys Compd. 509, 966–972 (2011)
W. Onreabroy, K. Papato, G. Rujijanagul, K. Pengpat, T. Tunkasiri, Ceram. Int. 38, S415–S419 (2012)
S.K. Pradhan, S. Bid, M. Gateshki, V. Petkov, Mater. Chem. Phys. 93, 224–230 (2005)
B. Timmer, W. Olthuis, A. van den Berg, Sens. Actuators B. 107, 666–677 (2005)
G.K. Mani, J.B. Rayappan, Sens. Actuators B Chem. 198, 125–133 (2014)
N.S. Chen, X.J. Yang, E.S. Liu, J.L. Huang, Sens. Actuators B Chem. 66, 178–180 (2000)
R. Kamble, B.V.L. Mathe, Sens. Actuators B Chem. 131, 205–209 (2008)
S. Ponmudi, R. Sivakumar, C. Sanjeeviraja, C. Gopalakrishnan, K. Jeyadheepan, Appl. Surf. Sci. 466, 703–714 (2019)
Acknowledgements
The author (S. Jerome Das) is grateful to the Management of Loyola College, Chennai-34 for awarding Loyola College TOI project (3LCTOI14PHY002) and acknowledges the technical help rendered by sophisticated Analytical Instrumentation Facility, Cochin for TEM and UV analysis. The authors are grateful to Dr. Jeyadheepan, Assistant Professor, Multifunctional Materials and Devices lab, Sastra Deemed University for Gas sensor studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deepapriya, S., Devi, S.L., Vinosha, P.A. et al. Estimating the ionicity of an inverse spinel ferrite and the cation distribution of La-doped NiFe2O4 nanocrystals for gas sensing properties. Appl. Phys. A 125, 683 (2019). https://doi.org/10.1007/s00339-019-2978-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-019-2978-x