Abstract
An argyrodite type Li6PS5Cl was prepared by the solution process using a mixture of solvents with a fast evaporation rate. The crystal phase and ionic conductivity of the Li6PS5Cl solid electrolyte were examined by X-ray diffraction and electrochemical impedance spectroscopy, respectively. Li6PS5Cl derived from solution process shows an argyrodite structure with an ionic conductivity of 6 × 10-5 S cm−1 at room temperature. Composite cathode was directly prepared by dispersing LiNi1/3Mn1/3Co1/3O2 (84 wt%) and a conductive additive (2 wt%) into a Li6PS5Cl precursor solution (14 wt%), with subsequent heating at 150 °C. Morphology of the composite cathode was evaluated by scanning electron microscopy and energy-dispersive X-ray spectroscopy. The formation of Li6PS5Cl-layer on the active material particles was observed. A bulk-type all-solid-state cell was fabricated with composite cathode derived from the solution process, achieving an initial discharge capacity of 160 mAh g−1 and capacity retention of ~80% after 20 cycles with a capacity efficiency of 100%.

Argyrodite type Li6PS5Cl prepared by solution process and its application to prepare composite cathode for an all-solid-state lithium battery.
Highlights
-
An argyrodite type Li6PS5Cl was prepared by solution process using a mixture solvents with a fast evaporation rate.
-
Composite cathode for all solid state battery was directly prepared by dispersing a cathode material in the precursor solution.
-
The cell worked as a rechargeable battery with good cycle performance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
All-solid-state lithium secondary batteries (ASLBs) using inorganic solid electrolytes have been predicted as next generation batteries with having higher energy density, safety and reliability. Sulfide-based solid electrolytes (SSE), such as glassy or glass-ceramic Li2S-P2S5 or Li2S-P2S5-GeS2 systems, have been widely used to fabricate bulk-type ASLBs [1,2,3,4], not only because of their high lithium ion conductivity (10−2–10−4 S cm−1), but also because of their mechanical properties that allow an intimate contact between electrode and electrolyte [5]. Usually, composite cathode is prepared by a simple mixture of active material, solid electrolyte, and/or carbon additives [1,2,3,4]. In general terms, the solid electrolyte content in the composite cathode obtained by simple mixture may vary from 30 to 60 wt% achieving up to 70% of the total theoretical discharge capacity (e.g., circa of 100 mAh g−1 for LiCoO2 [1, 2]). To obtain superior electrochemical performance in ASLBs, it is necessary to prepare favorable interfaces between electrodes and solid electrolytes with large contact areas. As liquids easily cover the surface of a solid, the favorable interfaces between electrodes and solid electrolytes can be prepared by the coating of a liquid precursor of the solid electrolyte on the electrode material by solution phase process and subsequent drying and heating to form solid electrolyte from the precursor. Recently, SSEs have been prepared by solution process achieving high ionic conductivities of 10−4–10−5 S cm−1. Some examples include Li3PS4 (2.3 × 10−6 S cm−1) [6], LiI-Li4SnS4 (4.1 × 10−4 S cm−1) [7], Li4SnS4 (1.4 × 10−4 S cm−1) [8], Li6PS5Br (3.4 × 10−5 S cm−1) [9], Li6PS5Cl (1.4 × 10−5 S cm−1) [10, 11], from different solvents such as N-methylformamide [6], methanol [7], water [8], ethanol [10] or solvent mixtures, ethanol, and ethyl propionate [9]. The composite cathodes were obtained after the solvent removal at relative low temperatures of 80–200 °C [7, 9] or higher temperature up to 320 °C [8] to promote higher ionic conductivity of SSE-layer. The formation of an adequate interface between SSE-layer and active material has been verified by the good electrochemical performance of the bulk-type ASLBs. Composite cathodes with ca. 15 wt% SSE-layer have achieved high initial discharge capacities of 110 mAh g−1 (LiNi1/3Mn1/3Co1/3O2) [9] and 140 mAh g−1 (LiCoO2) [7, 8].
Based on these positive achievements, in the present work, we studied a new solvent medium to prepare a SSE-layer and promote a better interface between active material and sulfide solid electrolyte for a bulk-type ASLB. In the formulation of the coating, the selection of solvents is based on their evaporation rate because the evaporation rate plays an important role in obtaining adequate coating properties such as density, covering degree, and homogeneity [12]. In a previous study, Li6PS5Cl was dissolved in ethanol and it was used to produce a SSE-layer [10, 11]. Here, a solvent medium combining ethyl acetate and ethanol is proposed to control the properties of the sulfide electrolyte layer since both solvents are fairly volatile [12] with low boiling points around 78 °C. In addition, both solvents have relatively low toxicity and low cost making them suitable for a further industrial application. To evaluate the effectivity of the solvent medium to form an appropriate SSE-layer, a composite cathode was prepared using Li6PS5Cl and high voltage cathode LiNi1/3Mn1/3Co1/3O2.
2 Experimental
All of the experiments in this study were conducted under an argon atmosphere (H2O ≤ 5, O2 ≤ 1 p.p.m).
2.1 Synthesis of composite cathode
The composite cathodes were prepared by solution process using LiNbO2-coated LiNi1/3Mn1/3Co1/3O2 (NMC) [13, 14], Li6PS5Cl solid electrolyte and vapor grown carbon fiber (VGCF, Showa Denko, Japan) as the active material, ionic, and electronic conductor additives, respectively. Super-dehydrated ethanol (99.5%, Wako Pure Chemical, Japan) and super-dehydrated ethyl acetate (99.5%, Wako Pure Chemical, Japan) were used as solvents. The weight ratio of NMC:Li6PS5Cl:VGCF of 84:14:2 was evaluated.
Li6PS5Cl solid electrolyte was firstly prepared by a mechanical milling process following the procedure reported previously [11]. Typically, 0.5 g of stoichiometric proportions of Li2S (Mitsuwa’s Purity Chemicals, 99.9%), P2S5 (Sigma Aldrich, 99%), and LiCl (Sigma Aldrich, 99.9%) were milled with 15 zirconia balls (10 mm diameter) in a zirconia pot (45 mL) using a planetary ball mill (PULVERISETTE, Fritsch, Germany) for 40 h at 600 r.p.m. The as-synthetized Li6PS5Cl solid electrolyte powder with an ionic conductivity of 4 × 10−5 S cm−1 was then used to prepare the SSE-solution and further, Li6PS5Cl-layer on active material. Li6PS5Cl powder was dissolved in a mixture of ethanol and ethyl acetate (molar ratio of 4:6), the concentration of Li6PS5Cl solution was 0.1 g mL−1. The composite cathode was finally prepared by solution process dispersing NMC and VGCF in the Li6PS5Cl-solution. A vortex mixer at 2800 r.p.m. (AS ONE test tube shakers, Japan) was used to homogenize the slurry and the solvents were then removed by heating at 150 °C under vacuum.
The crystal phase, morphology, and ionic conductivity of the Li6PS5Cl solid electrolyte obtained by dissolution-reprecipitation process were examined to elucidate the effect of the solvents on the properties of the sulfide electrolyte. The crystal phase evaluated by X-ray diffraction (XRD) was carried out with an X-ray diffractometer (MultiFlex600, Rigaku) using CuKα radiation. In XRD measurements, a stainless-steel background holder covered with a protective Kapton film was used to prevent reaction of solid electrolyte with moisture. The ionic conductivity of pelletized samples was determined by electrochemical impedance spectroscopy (EIS) using an impedance analyzer (SI 1260, Solartron) in the frequency range of 0.1 MHz to 200 Hz at the amplitude of 10 mV. The solid electrolyte powders (110 mg, thickness of ~0.9 mm) were pressed under 360 MPa (at room temperature) in a polycarbonate tube, 10 mm of diameter and two stainless steel (SS) disks were used as current collectors. The sample was placed in a sealed glass container with terminals in a glove box. The impedance was measured under the inert conditions. Morphology and compositional distribution of the prepared composite cathode were studied by scanning electron microscopy (SEM), performed on a JIB-4600F Multibeam SEM-FIB Scanning Electron Microscope.
2.2 Cell assembly and electrochemical measurements
The all-solid-state battery was constructed using 80Li2S·20P2S5 (mol%) glass and indium metal (99.99% 0.1 mm thickness) as solid electrolyte and anode, respectively. The 80Li2S·20P2S5 solid electrolyte was prepared by mechanical milling using 1 g of stoichiometric proportions of Li2S and P2S5 and 4 mm diameter zirconia balls (500 balls) in a zirconia pot (45 mL) at 510 r.p.m. for 10 h. The composite cathode (10 mg) and the solid electrolyte powder (80 mg) were pressed under 360 MPa in a polycarbonate tube (ɸ = 10 mm). The indium metal (40 mg) was pressed under 240 MPa on the prepared pellet. The three-layered pellet was sandwiched between two stainless-steel disks as current collectors to fabricate two electrode cells. The all-solid-state cells were placed in a sealed glass container with terminals, in a glove box. The all-solid-state cells were evaluated under the inert conditions. The electrochemical performance of the cell was evaluated under a constant current density of 0.13 mA cm−2 in the voltage range from 2.0 V to 3.8 V vs. Li-In at room temperature, using a charge discharge measuring device (Scribner Associates, 580 battery type system).
An all-solid-state battery fabricated with composite cathode prepared by a simple mixture of NMC, Li6PS5Cl obtained by mechanical milling and VGCF was used as a comparison. NMC, Li6PS5Cl, and VGCF were mixed using a vortex mixer with the same mass ratio of the composite cathode prepared by solution process.
3 Results and discussion
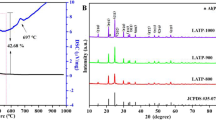
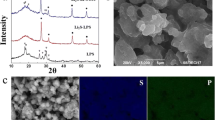
Figure 1a shows a photography of Li6PS5Cl solution. Homogeneous yellow solution was obtained after full dissolution of Li6PS5Cl prepared by mechanical milling in a mixture of ethanol and ethyl acetate solvents. Figure 1b displays the X-ray diffraction (XRD) patterns of the Li6PS5Cl solid electrolyte obtained by mechanical milling and dissolution-reprecipitation procedures. The precipitation of Li6PS5Cl powder from Li6PS5Cl-SSE-solution was promoted at 150 °C. The XRD patterns at two different period times of heat treatment were included. The indexed XRD pattern of the Li6.2PS5Cl (ICDD #418490) phase was also included for comparison. The Li6PS5Cl phase is clearly observed in the XRD pattern of the powder obtained by mechanical milling, indicating a complete reaction of raw materials. The XRD pattern of the Li6PS5Cl powder obtained by dissolution-reprecipitation process shows the Li6PS5Cl phase as the major phase, a small peak around 35° is attributed to the LiCl. Small additional reflections are observed in the XRD pattern of the Li6PS5Cl powder obtained after heating at 150 °C for 1 h. These reflections could not be indexed by the structure of the starting precursor materials, however, they disappear when the heat treatment was extended to 3 h. The formation of complex between solid electrolyte and solvents could produce these additional reflections [15]. The XRD results indicate that the solvents do not produce significant changes in the structure of Li6PS5Cl crystal phase obtained by dissolution-reprecipitation process.
Figure 2 shows the impedance plot of Li6PS5Cl obtained by the dissolution-reprecipitation process. The Nyquist plot consists in a semicircle at high frequency and a capacitive tail at low frequency due to the interface between the ionic conductor and blocking stainless steel electrodes. The total resistance (bulk and grain boundary resistances) used to calculate the ionic conductivity was estimated at the intersection point, Zˈ axis, between semicircle and capacitive tail (3 × 104 Hz). Ionic conductivity of the Li6PS5Cl attains 6 × 10−5 S cm−1 at room temperature. The high crystallinity of Li6PS5Cl obtained by the dissolution-reprecipitation process, verified by XRD patterns (Fig. 1b), make it difficult to pelletize the sample leading to a possible underestimated ionic conductivity. The ionic conductivity of the Li6PS5Cl obtained by dissolution-reprecipitation process was lower than that obtained by mechanical milling and heated at 550 °C (1 × 10−3 S cm−1 [16, 17]), and slightly higher to the Li6PS5Cl obtained by solution process from ethanol solution (~2 × 10−5 S cm−1) [10, 11]. In crystallized samples, the high thermal treatment is used to create a better grain boundary contact between particles [17]. The lower ionic conduction of SSE derived from solution process has been attributed to an increase of the grain boundary resistance produced most likely by the remaining organic compounds on the surface of SSE particles [10, 15]. In this sense, the unassigned peaks observed in the XRD pattern of Li6PS5Cl crystal derived from Li6PS5Cl solution at 150 °C for 1 h (Fig. 1b), suggest that the presence of remaining organic phases in the Li6PS5Cl are promoted by insufficient thermal treatment. In our previous results [11], a Li6PS5Cl crystal derived from ethanol showed a well crystallized argyrodite phase without any additional peak after solvent evaporation at lower temperature of 80 °C. The boiling point and vapor pressure of both, ethanol (~5.95 kPa at 20 °C) and ethyl acetate (~9.73 kPa at 20 °C), are low and quite similar, therefore these should be evaporating at low temperature around 80 °C. However, a higher temperature of 150 °C was needed to completely remove ethanol and ethyl acetate, used to prepare the Li6PS5Cl crystal reported in this work, evidencing a different reactivity of Li6PS5Cl with the solvents. The chemical interactions between the Li6PS5Cl and organic solvent medium is still not understood and further studies will be carried out to elucidate this behavior.
A heat treatment at relative low temperature of 150 °C during a short period time of 3 h is suitable to promote the entire solvents removal to prepare Li6PS5Cl-layer on NMC particles with moderate-high ionic conductivity.
Figure 3 displays the SEM morphology of composite cathode obtained by liquid phase process. EDS mapping images of cobalt, sulfur, and chlorine are also shown. The SEM image discloses NMC particles with a size around 5 µm. The sulfur and chlorine, elements from the sulfide solid electrolyte, were detected on the surface of active material particles. Although coating on NMC secondary particles with aggregates of primary particles is very challenging, the liquid phase process resulted to be effective to produce a good distribution of Li6PS5Cl-layer on them.
Figure 4 shows charge-discharge curves and cycle performance of the bulk-type ASLB cell fabricated with composite cathode prepared by the liquid phase process. The composite cathode contains a relative low content of SSE of 14 wt%. The all-solid-state cell was evaluated in a voltage range of 2 V to 3.8 V (vs. Li-In) at a current density of 0.13 mA cm−2 at 25 °C. The cell works as a rechargeable battery achieving an initial discharge capacity of 160 mAh g−1 and capacity retention of ~80% after 20 cycles with a capacity efficiency of 100%. For comparison, Fig. 5 shows charge-discharge curves and cycle performance of the all-solid-state cell fabricated with composite cathode prepared by a simple mixing of solid electrolyte powder and the active material. The Li6PS5Cl obtained by mechanical milling with an ionic conductivity of 4 × 10−5 S cm−1 was used to prepare the composite cathode. The content of Li6PS5Cl was 14 wt%, which is the same as that prepared by the solution process. The cell works as a rechargeable battery achieving a lower initial discharge capacity of 92 mAh g−1 and capacity retention of ~80% after 20 cycles with a capacity efficiency of 100%.
The high initial discharge capacity of the bulk-type ASLB cell with the composite cathode prepared by solution process indicates that the Li6PS5Cl-layer is well formed facilitating an adequate ionic conduction to the active material, better than using a simple mixture process. The conductivity of Li6PS5Cl used in both composite cathodes is quite similar, 4–6 × 10−5 S cm−1, and thus the difference of their electrochemical performance is associated directly to the improvement of the interface between electrode and electrolyte obtained by solution process.
The initial discharge capacity of the bulk-type ASLB cell prepared by solution process is also higher than reported by other composite cathodes prepared by solution process with the same composition using argyrodite-type Li6PS5X (Cl, Br) as a SSE-layer and NMC as active material and, ethanol or mixture of ethanol and ethyl propionate as solution medium [9, 11]. Composite cathode derived from ethanol solution shows an initial discharge capacity of 44 mAh g−1 [11], while 110 mAh g−1 has been reported from a solvent mixture of ethanol and ethyl propionate [9]. Although, the interaction between sulfide solid electrolyte and solvents is still unclear, the different electrochemical performance of these composite cathodes obtained by solution process evidences that this interaction can be modified depending on the solvent or their mixture. Difficulty in removal is expected with ethyl propionate since it has a slightly high boiling point of 99 °C compared with ethyl acetate.
Regarding cycle performance of the bulk-type ASLB cell with the composite cathode prepared by solution process, gradual decrease of the discharge capacity is attributed to the reaction in the interface between Li6PS5Cl-SSE-layer and active material. In fact, the discharge capacity diminishes from 160 mAh g−1 to 140 mAh g−1 after the first and second cycle, evidencing a high reactivity during the first cycles. After the second cycle, the discharge capacity decreases slightly attaining a good capacity efficiency of 100%.
4 Conclusions
Argyrodite type Li6PS5Cl was prepared by solution process using a mixture of ethanol and ethyl acetate as solvent medium. Crystal phase of Li6PS5Cl was confirmed after a complete removal of the solvents at 150 °C for 3 h. Li6PS5Cl solution was used to prepare composite cathode by dispersion of LiNi1/3Mn1/3Co1/3O2 and carbon particles, followed by solvent elimination at 150 °C. A bulk-type all-solid-state battery fabricated with the composite cathode obtained by solution process showed a high initial discharge capacity of 160 mAh g−1. Solution process has proven to be effective to produce a good interface between Li6PS5Cl-layer and active material. The selection of adequate solution medium could improve the electrochemical performance of composite cathode obtained by solution process.
5 Disclaimer
The material has not been published in whole or in part elsewhere; The paper is not currently being considered for publication elsewhere; All authors have been personally and actively involved in substantive work leading to the paper, and will hold themselves jointly and individually responsible for its content.
References
Sakuda A, Kitaura H, Hayashi A, Tadanaga K, Tatsumisago M (2008) Improvement of high-rate performance of all-solid-state lithium secondary batteries using LiCoO2 coated with Li2O-SiO2 glasses. Electrochem Solid State Lett 11(1):A1–A3. https://doi.org/10.1149/1.2795837
Auvergniot J, Cassel A, Ledeuil JB, Viallet V, Seznec V, Dedryvere R (2017) Interface stability of argyrodite Li6PS5Cl toward LiCoO2, LiNi1/3Co1/3Mn1/3O2, and LiMn2O4 in bulk all-solid-state batteries. Chem Mater 29(9):3883–3890. https://doi.org/10.1021/acs.chemmater.6b04990
Oh G, Hirayama M, Kwon O, Suzuki K, Kanno R (2016) Bulk-type all solid-state batteries with 5V class LiNi0.5Mn1.5O4 cathode and Li10GeP2S12 solid electrolyte. Chem Mater 28(8):2634–2640. https://doi.org/10.1021/acs.chemmater.5b04940
Zhang Q, Mwizerwa JP, Wan HL, Cai LT, Xu XX, Yao XY (2017) Fe3S4@Li7P3S11 nanocomposites as cathode materials for all-solid-state lithium batteries with improved energy density and low cost. J Mater Chem A 5(45):23919–23925. https://doi.org/10.1039/c7ta07972a
Hayashi A, Sakuda A, Tatsumisago M (2016) Development of sulfide solid electrolytes and interface formation processes for bulk-type all-solid-state Li and Na batteries. Front Energy Res 4. https://doi.org/10.3389/fenrg.2016.00025
Teragawa S, Aso K, Tadanaga K, Hayashi A, Tatsumisago M (2014) Liquid-phase synthesis of a Li3PS4 solid electrolyte using N-methylformamide for all-solid-state lithium batteries. J Mater Chem A 2(14):5095–5099. https://doi.org/10.1039/c3ta15090a
Park KH, Oh DY, Choi YE, Nam YJ, Han LL, Kim JY, Xin HL, Lin F, Oh SM, Jung YS (2016) Solution-processable glass LiI-Li4SnS4 superionic conductors for all-solid-state Li-ion batteries. Adv Mater 28(9):1874–1883. https://doi.org/10.1002/adma.201505008
Choi YE, Park KH, Kim DH, Oh DY, Kwak HR, Lee YG, Jung YS (2017) Coatable Li4SnS4 solid electrolytes prepared from aqueous solutions for all-solid-state lithium-ion batteries. Chemsuschem 10(12):2605–2611. https://doi.org/10.1002/cssc.201700409
Chida S, Miura A, Rosero-Navarro NC, Higuchi M, Phuc NHH, Muto H, Matsuda A, Tadanaga K (2017) Liquid-phase synthesis of Li6PS5Br using ultrasonication and application to cathode composite electrodes in all-solid-state batteries. Ceram Int 44(1):742. https://doi.org/10.1016/j.ceramint.2017.09.241
Yubuchi S, Teragawa S, Aso K, Tadanaga K, Hayashi A, Tatsumisago M (2015) Preparation of high lithium-ion conducting Li6PS5Cl solid electrolyte from ethanol solution for all-solid-state lithium batteries. J Power Sources 293:941–945. https://doi.org/10.1016/j.jpowsour.2015.05.093
Rosero-Navarro NC, Kinoshita T, Miura A, Higuchi M, Tadanaga K (2017) Effect of the binder content on the electrochemical performance of composite cathode using Li6PS5Cl precursor solution in an all-solid-state lithium battery. Ionics 23(6):1619–1624. https://doi.org/10.1007/s11581-017-2106-x
Smith RL (1984) Review of glycol ether and glycol ether ester solvent used in the coating industry. Environ Health Perspect 57:1–4. https://doi.org/10.2307/3429892
Sakuda A, Takeuchi T, Kobayashi H (2016) Electrode morphology in all-solid-state lithium secondary batteries consisting of LiNi1/3Co1/3Mn1/3O2 and Li2S-P2S5 solid electrolytes. Solid State Ion 285:112–117. https://doi.org/10.1016/j.ssi.2015.09.010
Ohta N, Takada K, Sakaguchi I, Zhang LQ, Ma RZ, Fukuda K, Osada M, Sasaki T (2007) LiNbO3-coated LiCoO2 as cathode material for all solid-state lithium secondary batteries. Electrochem Commun 9(7):1486–1490. https://doi.org/10.1016/j.elecom.2007.02.008
Calpa M, Rosero-Navarro NC, Miura A, Tadanaga K (2017) Instantaneous preparation of high lithium-ion conducting sulfide solid electrolyte Li7P3S11 by a liquid phase process. RSC Adv 7(73):46499–46504. https://doi.org/10.1039/C7RA09081A
Deiseroth HJ, Kong ST, Eckert H, Vannahme J, Reiner C, Zaiß T, Schlosser M (2008) Li6PS5X: a class of crystalline Li-rich solids with an unusually high Li+ mobility. Angew Chem, Int Ed 47(4):755–758. https://doi.org/10.1002/anie.200703900
Yu C, van Eijck L, Ganapathy S, Wagemaker M (2016) Synthesis, structure and electrochemical performance of the argyrodite Li6PS5Cl solid electrolyte for Li-ion solid state batteries. Electrochim Acta 215:93–99. https://doi.org/10.1016/j.electacta.2016.08.081
Acknowledgements
The present work was supported by the Japan Science and Technology Agency (JST), Advanced Low Carbon Technology Research and Development Program (ALCA), and Specially Promoted Research for Innovative Next Generation Batteries (SPRING) project. The analysis of SEM was carried out with JIB4600F at the “Joint-use Facilities: Laboratory of Nano-Micro Material Analysis”, Hokkaido University, supported by “Material Analysis and Structure Analysis Open Unit (MASAOU)”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Rosero-Navarro, N.C., Miura, A. & Tadanaga, K. Preparation of lithium ion conductive Li6PS5Cl solid electrolyte from solution for the fabrication of composite cathode of all-solid-state lithium battery. J Sol-Gel Sci Technol 89, 303–309 (2019). https://doi.org/10.1007/s10971-018-4775-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-018-4775-y