Abstract
Li3PS4(LPS) was in situ formed at the active material Li2S through the reaction of Li2S and P2S5 by mechanical ball milling, which can be used to composite cathode for Li/S battery. The fine active materials-electrolyte interface will be generated via in situ reaction. Compared with the direct mixing Li2S + LPS, the interface impedance between Li2S and LPS of Li2S-LPS had a significant decrease from 833.3 Ω to 203.7 Ω at room temperature. The effect of heat treatment temperature on the ionic conductivities of Li2S-LPS has been studied, and the Li2S-LPS-300 showed the highest ionic conductivity of 2.67 × 10−4 S cm−1 at room temperature. The all-solid-state cell (Li/LPS/Li2S-LPS) possessed a larger capacity and better rate performance than that of the cell using Li2S + LPS as cathode material, showing a discharge capacity of 624.5 mAh g−1 (g of Li2S) for the 20th cycle. We also find that high carbon content of composite cathode will reduce the utilization of Li2S. The composite cathode 90Li2S-LPS + 10C (wt%) shows the highest capacity of 763.8 mAh g−1 at the current density of 0.1 mA cm−2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the past few decades, lithium batteries have been wildly used in various fields in consideration of their high-energy density, long cycle life, and low self-discharge rate [1, 2]. Recently, with rapid development in electric vehicles, a larger amount of attention has been focused on lithium batteries for service as a large-scale power source [3]. However, currently commercialized lithium-ion batteries (LIB) with common organic liquid electrolytes usually suffer from serious safety issue [4, 5]. Moreover, the necessary increase of the rate performance and energy density of the next generation batteries will be facilitated by using the correct chemical strategies. All-solid-state lithium batteries, replacement of organic liquid electrolytes by inorganic solid electrolytes, have a good solution to the safety performance of the batteries. Because of the incombustibility of solid-state electrolytes, all-solid-state lithium batteries are recognized as prospective next-generation battery technologies [6]. The working voltage and energy density of the whole system can be significantly improved when the lithium metal anodes are matched [7]. Meanwhile, all-solid-state Li/S batteries have appealed to much notice on account of their favorable interface compatibility between electrolyte and cathode materials, high theoretical capacity, and chemical stability, and there is no shuttle effect with solid electrolytes [8, 9].
Great efforts have been reported to improve the ionic conductivity of solid electrolytes, and several inorganic solid electrolytes with high conductivity have been studied. Many works have been carried out for the sulfide electrolytes because of their higher ionic conductivity (>10−3 S cm−1), low-temperature preparation processing, better stability against lithium metal, and appropriate mechanical properties than oxides. The preparation process of the high-conduction sulfide electrolytes has been widely reported, including ball milling conditions, quenching, and intricate sintering process. The ionic conductivity of some sulfide solid electrolytes has been equivalent to that of organic electrolytes, which can meet the requirements of application [10, 11].
As we know, both S and Li2S have very low electron and ionic conductivity. Therefore, it is necessary to mix conductive carbon and sulfur solid electrolyte into the composite cathode of all-solid-state lithium-sulfur battery to construct effective transport path of electrons and ions. Grinding and ball milling are common methods to prepare composite cathode. In this respect, some solid batteries have been examined. However, the poor interfacial contact of each component in composite cathode is the dominating obstacle to the large-scale practical using all-solid-state batteries [12, 13]. Therefore, it is necessary to develop an effective way for improving close interface contact between active materials and solid electrolytes. A highly Li3PS4 solid electrolyte has been coated onto nano-Li2S as lithium superionic sulfide cathode by a liquid-phase method [14, 15]. However, the lithium superionic sulfide cathode ionic conductivity of 10−6S cm−1 requires further advance for meeting applications.
Herein, we reported a Li2S-LPS composite cathode through the reaction of Li2S and P2S5 by mechanical ball milling, and the Li2S-LPS composite was then mixed with acetylene black (AB) to form composite cathode material. The in situ formed LPS can intimate on the Li2S particles, which significantly reduce the interface impedance between active material and electrolyte in composite cathode. The effect of heat treatment temperature on the ionic conductivities of obtained Li2S-LPS has also been researched. Compared with Li2S + LPS prepared by ball mixing, all-solid-state cells employing Li2S-LPS as cathode exhibit excellent cycle performance, high rate performance, and high utilization of active materials. In addition, it was found that high carbon content in composite cathode not only reduces the content of active substance but also reduces the utilization of Li2S.
Experimental section
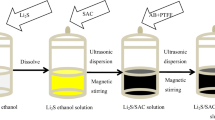
Synthesis of Li2S-LPS and Li2S + LPS
The Li2S-LPS (90.5Li2S-9.5P2S5, mol%) glass-phase material was compounded by high-energy ball milling (Fritsch Pulverisette 7) of Li2S (Alfa, 99.9%) and P2S5 (Aladdin, 99.9%) using Al2O3 pots (volume is 45 mL) with 12 Al2O3 balls (10-mm diameter). The condition of the ball milling was set as 510 rpm for 8 h. An intermediate cooling time of 20 min was allowed between every 40-min ball milling. The mass ratio of Li2S and LPS in Li2S-LPS is 45:55 after the reaction of P2S5 and Li2S in the raw material. Then, the glass powders were transformed into the glass-ceramic samples by simple heat treatment without other intricate sintering procedure. The amorphous phase powders were transferred to quartz tube and heated at 260, 280, 300, and 320 °C for 1 h, respectively. Among, the heating rate was controlled at 5 °C/min. The obtained glass-ceramic powders by different heat treatment temperature are named Li2S-LPS-260, Li2S-LPS-280, Li2S-LPS-300, and Li2S-LPS-320. It is worth noting that all procedures were implemented under argon atmosphere protection to prevent the sulfide solid electrolyte to moisture.
The sulfide solid electrolyte LPS(75Li2S-25P2S5) was synthesized by Li2S (Alfa, 99.9%) and P2S5 (Aladdin, 99.9%). The LPS (75Li2S-25P2S5) glass-phase powder was prepared at 510 rpm for 8 h, and then the mixture was heated up to 260 °C for 1 h at a rate of 5 °C/min. The obtained glass-ceramic powders labeled as LPS. The Li2S + LPS was consisted by the Li2S and LPS with the weight ratio of 45:55 and compounded by mechanical milling at 380 rpm for 8 h. An intermediate cooling time of 20 min was allowed between 40 min ball mill. All procedures were implemented under argon atmosphere protection.
Characterization of materials
X-ray diffraction (RIGAKUTIR-3) measurements were used to characterize the structure of the Li2S-LPS powder, the LPS powder, and the composite cathode pellets with Cu Kα radiation at room temperature. XRD data were recorded in the range of 2θ = 10~60o with a scan speed of 7°min−1. To prevent moisture, the samples were put into the quartz sample tank and covered with polyimide film. Raman spectra of the samples were measured with confocal Roman spectrometer (Renishaw in via, UK) using the 532 nm line of semiconductor laser beam. The morphologies and EDX analysis mapping of sample were observed by scanning electron microscopy (SEM) (HITACHIS-4800). X-ray photoelectron spectroscopy (XPS) (ESCALAB 250) was used to examine the surface chemical composition of Li2S-LPS and the composite cathode materials. In order to prepare the sample for XPS test, the cell was charged and discharged to a specified voltage, and the composite cathodes were then taken out. All samples transferred into the XPS chamber under argon atmosphere protection.
Electrochemical performances testing
Electrochemical impedance spectroscopy (EIS) was employed to characterize the transport properties of the samples. Firstly, the powder samples were pressed into pellets with a diameter of 10 mm and a thickness of 0.8 mm. Then gold plated on both sides of the pellets was used as blocking electrode by ion sputtering. Finally, two pieces of stainless steel sheets were used as collectors to clamp the pellets. The impedance of selected cells was measured from 1 MHz to 0.01 Hz at room temperature.
The composed electrodes of Li2S-LPS-300 and acetylene black (AB) with the weight ratio of 75:25, 80:20, 85:15, and 90:10 were prepared by mechanical milling with a planetary ball mill apparatus at 380 rpm for 3 h, which were directly used as a working electrode. The composite cathode prepared was named Li2S-LPS + 25C, Li2S-LPS + 20C, Li2S-LPS + 15C, and Li2S-LPS + 10C, respectively. As for cells using Li2S as active material, the composed electrodes of Li2S + LPS and acetylene black (AB) with the weight ratio of 80:20 were prepared using the same ball milling conditions. Bilayer pellets were formed by pressing the SE powders (100 mg) and the composed electrodes (3 mg) at 480 MPa (10 mm diameter). The mass loading of cathode was ~ 3.82 mg cm−2, corresponding to the Li2S loading of 1.38 mg cm−2. Then, a piece of lithium was then attached to SE side of the bilayer pellet by pressing at 190 MPa. Two stainless steel disks as the current collector were attached to the three-layered pellets. The cell with 20% carbon content was cycled at a constant current density of 0.050 mA cm−2 at 60°C in a constant-temperature chamber, while the cell with different carbon content was cycled at a constant current density of 0.1 mA cm−2. Cycle voltammograms (CV) were measured between 0.8 V and 4.0 V at a scan rate of 0.1 mV s−1.
Result and discussion
Figure 1a showed XRD patterns of the LPS powder, Li2S-LPS glass powder, and Li2S-LPS-300 powder, respectively. The XRD patterns of the LPS powder showed that there have six typical diffraction peaks at about 2θ = 17.7°, 18.3°, 20.0°, 26.1°, 29.4°, and 30.0°, which are assigned to LPS. Furthermore, only the characteristic diffraction peaks of Li2S appear in the Li2S-LPS amorphous sample, and no diffraction characteristics peaks assigned to LPS. The XRD spectrum of Li2S-LPS-300 is similar to that of Li2S-LPS amorphous sample. It is worth emphasizing that the LPS phase with obvious diffraction peaks about 17.7°, 18.3°, 20.0°, and 29.4° was formed and observed after heat treatment. In addition, no characteristic diffraction peaks of P2S5 were found in the XRD spectra of LPS and LPS-300. It is inferred that the sample consists of LPS and Li2S and all the starting materials P2S5 reacted with Li2S to transform into LPS. Figure S3 (Supporting Information) shows the Roman spectra of Li2S, LPS, Li2S + LPS, and Li2S-LPS. Only one peak was observed at 420 cm−1 attributed to PS43−in the spectrum of the LPS. And one peak was observed at 373 cm−1 assigned to Li-S-Li in the spectrum of the Li2S. The Roman spectrum of Li2S + LPS is similar to that of Li2S-LPS; both peak at 420 cm−1 attributed to PS43− and peak at 373 cm−1 assigned to Li-S-Li were observed. It was revealed that both Li2S + LPS and Li2S-LPS consist of LPS and Li2S.
Figure 1b and 1c showed the scanning electron microscopy (SEM) and energy dispersive X-ray analysis (EDS) mapping (P and S) images of sample Li2S-LPS-300. Sample Li2S-LPS-300 consists of micrometer primary particles and amorphous phase binding of the primary particles. Because of the severe agglomeration between the particles, it was difficult to measure the exact size of primary particles. The EDS mapping images showed that mapping areas of P overlapped the area of S, indicating that the excess Li2S in raw materials was fully contacted with the sulfide electrolyte LPS formed by ball milling. On the contrary, Li2S and LPS in Li2S + LPS prepared by direct ball milling have not been fully contacted in previous literatures [16].
To further determine chemical composition of the Li2S-LPS, XPS was used to analyze the surface of Li2S-LPS pellet. As shown in Fig 2, both the S2p and P2p core levels present 2p1/2 and 2p3/2 doublet peaks with two shake-up satellites. A trace of PS43−(2p3/2, 161.8 eV and 2p1/2, 163.0 eV) and S2−(2p3/2, 160.7 eV and 2p1/2, 161.9 eV) was detected in the Li2S-LPS composite. Moreover, only the trace of PS43−(2p3/2, 132.2 e and 2p1/2, 133.3 eV) was observed in the P2p core level. It is inferred that Li2S-LPS consists of Li2S and LPS, which is consistent with XRD analysis.
Li-ion conductivity of Li2S-LPS and Li2S + LPS was measured by electrochemical impendence spectroscopy (EIS) with two thin gold film electrodes at room temperature. All of the Nyquist plots were gathered in Fig. 2a. EIS curves of each sample show a single semicircle at high frequency and a tail in the low frequency; these two components can be related to the bulk/interface resistance and electrode contributions [17]. The equivalent circuit Rbulk(RIFQ1)Q2 (Q is a constant phase element, CPE) was used to fit EIS data and calculate the bulk resistance(Rbulk) and interface resistance(RIF), and the results were listed in Table 1. The RIF represents the overlap of interface impedance between Li2S and LPS, which was found to be 203.7 Ω and 833.3 Ω for sample Li2S-LPS and Li2S + LPS, respectively. EIS fitting results show that the interface impedance between Li2S and LPS of Li2S-LPS prepared by the one-step ball milling is much smaller than that of Li2S + LPS when the content of Li2S is the same. This indicated that the transport speed of Li+ at the Li2S/LPS interface constructed by in situ reaction is faster than that of directly mixed Li2S and LPS. But the explicit Li+ transmission mechanism in Li2S/LPS interface of Li2S-LPS is still under study.
Figure 3b exhibits the temperature dependence of the conductivity of Li2S-LPS with different annealing temperature. With the increased of sintering temperature from 26 to 300 °C, the ionic conductivity of the Li2S-LPS enhanced from 2.44 × 10−4 S cm−1 to 2.67 × 10−4 S cm−1 at room temperature. However, when the heat treatment temperature increased to 320 °C, the ionic conductivity of the obtained samples decreases slightly. The plots of log (σ) against 1000/T were found to be linear and well fit with the Arrhenius equation, σ = A exp(-Ea/RT), and the activation energy of Li2S-LPS-300 was calculated from plot to be about 11.80 kJ mol−1.
Figure 4 shows cyclic voltammogram (CV) curve of cell using Li2S-LPS-300 as composite at room temperature in the potential range from 0.8 V to 4.0 V at a scan rate of 0.1 mV S−1. Unlike typical CV curves of a liquid electrolyte Li-S battery, only one wide reduction peak near 2.47 V and one oxidation peak around 1.67 V were observed. It has been reported that the equilibrium phase in the Li/S system using solid electrolytes was varied from that in commercial organic electrolytes Li/S cell; only Li2S was generated in electrochemical reaction [18]. The only one reduction peak can be attributed to the S8 transformed into S2− that strongly supports the direct reaction mechanism of Li/S battery using solid electrolytes.
To understand the electrochemical reaction of composite cathode during charging and discharging, the composition of composite cathode after charging and discharging was analyzed by XPS, and the results were shown in Fig 5. A trace of PS43− (2p3/2, 161.8 eV and 2p1/2, 163.0 eV) and S8(2p3/2, 163.2 eV and 2p1/2, 164.3 eV) was detected in the charged composite cathode S2p core level [19]. Correspondingly, the peak of P-S bond of LPS still existed in the composite cathode S2p spectra after discharge, and the peak of S-Li bond (2p3/2, 160.4 eV and 2p1/2, 161.6 eV) was observed, while the peak of S-S bond of sulfur disappeared. At the same time, there was no significant change in the spectra of P2p after charging and discharging, except for the 132.5 eV peak (S-P bond) of LPS. It is inferred that the Li2S in the composite cathode delithiation formed elemental sulfur when charging and the opposite when discharging, which was consistent with the conclusion of CV analysis. It was noteworthy that the charge capacity of the battery has not yet reached the theoretical capacity and there should be residual Li2S in the composite cathode after charging. However, there are almost no characteristic peaks of Li2S in the XPS spectrum of the composite cathode after charging. In this regard, XRD was used to further analyze the composite cathode after charging, and the results show that some of the unloaded Li2S exist (Fig. S2, Supporting Information). The reason for the absence of Li2S characteristic peaks in XPS spectra of composite cathode after charging may be that the samples will inevitably be exposed to air during the transfer to the test chamber and Li2S will react with moisture in the air to deteriorate.
Figure 6 shows the cycle performance and rate performance of the solid Li/S cells using Li2S-LPS-300/Li2S-LPS as cathode materials. Cells were operated under 60 °C at the current density of 0.05 mA cm−2. In order to activate the active material Li2S, the first charging cutoff voltage was 4 V, which was 3.5 V in the subsequent cycle. As displayed in Fig. 6a, solid Li/S cells, Li2S-LPS-300-based electrode/LPS/Li, shows a discharge capacity of 405.5 mAh g−1 (g of Li2S) for the 5th cycle. While during the following several cycles, the specific capacity continued to increase with the Coulombic efficiency above 100%. A similar phenomenon in Li2S solid Li/S cells during the first few cycles was also researched by Eom and Zhang [17, 20]. One possible reason is that Li+ from lithium electrode is embedded in the vacancies formed during preparation process or the cycle process until the vacancy is filled. Because of this activation, the discharge capacity increases to 624.5 mAh g−1 for the 20th cycle. The same phenomenon was observed in solid Li/S cell using Li2S + LPS as active material.
Discharge-charge voltage profiles of the cell with cathode electrode of Li2S-LPS (a) and Li2S + LPS (b) at 5th, 15th, and 20th; rate performance of the cell using Li2S-LPS (c) and Li2S + LPS (d) as positive materials; cycle performance of the cell with different composite electrodes at 0.05 mA cm−2(e).
Figure 6e shows cycle performance and Coulombic efficiency of the solid Li/S cells. Compared with the Li2S-LPS composite electrode, the poor contact between Li2S and LPS could lead to a poor active material utilization and low discharge capacity. The Li2S + LPS composite electrode exhibited an initial specific discharge of 150 mAh g−1and a reversible specific capacity of 213.6 mAh g−1 after 20 cycles.
Profile 6(c) and (d) in Fig. 6 show charging and discharging curves of the solid Li/S cells using Li2S-LPS and Li2S + LPS as cathode material at different currents from 0.05 mA cm−2 to 0.40 mA cm−2. The Li2S-LPS composites exhibited 624.5, 608.9, 592.9, and 576.2 mAh g−1at 0.05, 0.10, 0.20, and 0.40 mA cm−2, respectively. And the Li2S + LPS composite showed 213.6, 207.0, 144.7, and 118.7 mAh g−1at 0.05, 0.10, 0.20, and 0.40 mA cm−2. These results suggest that rate performance of the cell using Li2S-LPS composites is better than that of cell using Li2S + LPS composites. The rate performance is closely related to the contact area to each component in the cathode composites. Therefore, in situ growth LPS on Li2S is more effective in forming favorable interface between Li2S and LPS in composite cathode than conventional powder mixing protocol.
Due to the low electronic conductivity of the active material Li2S, conductive carbon is usually mixed into the composite cathode. The impact of carbon content in composite cathode on electrochemical performance was also discussed. Previously, the carbon content of composite cathode in all-solid-state lithium-sulfur batteries based on Li2S cathode generally exceeded 20%. However, we found that the utilization rate of Li2S in composite cathode with higher carbon content was lower. Figure 7a compares the cyclic performance of composite cathode with different carbon content. The composite cathode 75 Li2S-LPS + 25C shows the lowest capacity of 290.1 mAh g−1, while the 90 Li2S-LPS + 10C exhibits the highest capacity of 763.8 mAh g−1. Moreover, when the carbon content in composite cathode increases to 30%, the cathode layer will fall off when the battery was assembled by cold pressing. As we all know, sulfide electrolytes have high elasticity, which is also the reason why all-solid-state batteries using sulfide electrolytes can be assembled by cold pressing. However, the elasticity of conductive carbon is lower than that of sulfide electrolyte. Therefore, when a high content of conductive carbon is mixed into the composite cathode, the composite cathode layer prepared by cold pressing can easily be stripped from the electrolyte layer. And when the content of conductive carbon decreases to 5%, the charge-discharge capacity of the composite cathode is almost zero. This is because so little conductive carbon could not build an effective conductive electronic network in the composite cathode.
Conclusion
In this work, a layer of LPS was in situ generated on the Li2S particles by high-energy ball milling, which not only effectively constructs the conduction net of composite cathode but also significantly reduces the interface impedance between Li2S and LPS in composite cathode electrode. The effect of heat treatment temperature on the ionic conductivity of the Li2S-LPS has been studied. When the temperature of annealing is at 260–300 °C, the ionic conductivity of samples was gradually enhanced from 2.44 × 10−4 S cm−1 to 2.67 × 10−4 S cm−1. However, when the heat treatment temperature was further increased to 320 °C, the ionic conductivity decreased. Compared with Li2S + LPS, the Li2S-LPS composite cathode exhibits higher capacity and better cycle stability and rate performance. It shows a discharge capacity of 624.5 mAh g−1 (g of Li2S) for the 20th cycle at the current density of 0.05 mA cm−2. In addition, the study found that carbon content of composite cathode has a great impact on the utilization of Li2S. The composite cathode 90Li2S-LPS + 10C (wt%) shows the highest capacity of 763.8 mAh g−1 at the current density of 0.1 mA cm−2. The improved cell performance was indicating that in situ growth of LPS on Li2S is more effective in forming favorable ion-conductive pathway to composite cathode than conventional powder mixing protocol. We provide a facile and effective strategy to improve the interface between active substance and electrolyte in composite cathode of all solid-battery.
References
Herfurth HJ (2008) Nature451(7179):652
Tarascon JM, Armand M (2001) Nature 414(6861):359
Li W, Yao H, Yan K, Zheng G, Liang Z, Chiang YM, Cui Y (2015) Nat Commun 6:7436
Ren Y, Chen K, Chen R, Liu T, Zhang Y, Nan CW (2016) J Am Ceram Soc 98(12):3603
Inoue T, Mukai K (2017) ACS Appl Mater Interfaces 9(2):1507 Are All-Solid-State Lithium-Ion Batteries Really Safe?-Verification by Differential Scanning Calorimetry with an All-Inclusive Microcell, 1515
Qian J, Henderson WA, Xu W, Bhattacharya P, Engelhard M, Borodin O, Zhang JG (2015) Nat Commun 6:6362
Hu YS (2016) Nat Energy 1(4):16042
Han F, Yue J, Fan X, Gao T, Luo C, Ma Z, Suo L, Wang C (2016) Nano Lett 16(7):4521 High-Performance All-Solid-State Lithium-Sulfur Battery Enabled by a Mixed-Conductive Li2S Nanocomposite, 4527
Nagao M, Hayashi A, Tatsumisago M (2011) Electrochim Acta 56(17):6055
Kwon O, Hirayama M, Suzuki K, Kato Y, Saito T, Yonemura M, Kamiyama T, Kanno R (2014) J Mater Chem A 3(1):438
Kato Y, Hori S, Saito T, Suzuki K, Hirayama M, Mitsui A, Yonemura M, Iba H, Kanno R (2016) Nat Energy 1:16030
Sakuda A, Hayashi A, Tatsumisago M (2010) Chem Mater 22(3):949
Takada K (2013) Langmuir 29(24):7538 Interfacial nanoarchitectonics for solid-state lithium batteries, 7541
Liu Z, Fu W, Payzant EA, Xiang Y, Wu Z, Dudney NJ, Kiggans J, Hong K, Rondinone AJ, Liang C (2013) J Am Chem Soc135(3):975 Anomalous high ionic conductivity of nanoporous β-Li3PS4, 978
Lin Z, Liu Z, Dudney NJ, Liang C (2013) ACS Nano 7(3):2829
Shin M, Gewirth AA (2019) Adv Energy Mater 9(26):1900938
Zhang W, Weber DA, Weigand H, Arlt T, Manke I, Schröder D, Koerver R, Leichtweiss T, Hartmann P, Zeier WG (2017) ACS Appl Mater Interfaces 9(21):17835 Interfacial Processes and Influence of Composite Cathode Microstructure Controlling the Performance of All-Solid-State Lithium Batteries, 17845
Agostini M, Aihara Y, Yamada T, Scrosati B, Hassoun J (2013) Solid State Ionics, 244:48 A lithium–sulfur battery using a solid, glass-type P2S5–Li2S electrolyte, 51
Zhang Z, Wu D-H, Zhou Z, Li G-R, Liu S, Gao X-P (2018) Sci China Mater 62(1):74
Eom M, Son S, Park C, Noh S, Nichols WT, Shin D (2017) Electrochim Acta 230:279
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 145 kb)
Rights and permissions
About this article
Cite this article
Jiang, H., Han, Y., Wang, H. et al. In situ generated Li2S-LPS composite for all-solid-state lithium-sulfur battery. Ionics 26, 2335–2342 (2020). https://doi.org/10.1007/s11581-019-03287-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-019-03287-9