Abstract
The stabilization of metal nanoparticles using surface modification has been intensively investigated. We propose an alternative to the use of surfactants, long-chain polymers or silica shells in order to provide easy and efficient stabilization of a wide range of metallic nanostructures. The prepared silicon oligomers were characterized, optimized and successfully used for surface modifications of nanospheres, nanobipyramids, nanorods of both gold and silver. The modified nanoparticles were then easily incorporated into monolithic sol–gel materials based on silica. This original route toward hybrid composite was efficiently used to prepare composite sol–gel materials with plasmonic nanostructures for optical applications.
Graphical Abstract
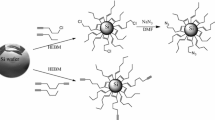
Specifically designed silicon polymers are used to efficiently stabilize metal nanoparticles and allow homogeneous dispersion into sol–gel materials.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Surface modification of metal nanoparticles has been investigated for a long time with the aim to either contribute to the stability of the colloids or to achieve new functionalities such as fluorescence or molecular recognition. Incorporation of metal nanoparticles in transparent glass [1, 2] or polymers [3, 4] has also been investigated for optical application but remains complicated due to particles instability during the preparation process, and to a difficult control of the shape/size of the nanostructures. In situ growth of metal nanoparticles in glass matrices can be achieved through several processes such as laser, thermal or chemically induced growth but remain limited to mostly spherical nanoparticles or wires-type nanostructure and generally with poor homogeneity [1–22]. The use of polymers for colloidal stabilization has already been described in the case of metal nanoparticles and in particular gold nanoparticles (AuNPs). Thus, various systems have been studied such as thiolated polyethyleneglycol (PEG-SH) [23–26], polyelectrolytes [27–29], polyelectrolytes combined with silica shell and hydrophobation using octadecyltrimethoxysilane [30], mercaptosuccinic acid followed by TOAB [31] or mercaptopropyltrimethoxysilane followed by hydrophobation by octadecyltrimethoxysilane [32]. Despite the important amount of studies, the obtained systems are either complicated to achieve or do not offer the desired stability in organic solvent allowing their use during sol–gel process.
We propose an original approach, which involves the surface modification of metal nanoparticles of various shapes and sizes using a functional thiolated silicon polymer. This polymer acts as a stabilizing agent of the colloids both in wide range of solvent and in transparent glass materials using the sol–gel process. In this paper we intend to develop the synthesis and optimization of the silicon polymer structure and the surface modification of gold nanostructures.
2 Experimental details
2.1 Synthesis of silicon polymer P1
8.3 mL (46.95 mmol) of diethoxydimethylsilane, 760 µL (4.18 mmol) of (3-mercaptopropyl)-methyl-dimethoxysilane and 780 µL (3.62 mmol) of (3-glycidoxypropyl)-methyl-dimethoxysilane (GLYDMO) were mixed together in a round bottom flask, equipped with a condenser, containing 4 mL MilliQ water and 40µL HCl (0.1 M). After 20 min of stirring at room temperature, 380 µL of triethylamine (2.7 mmol) was added. The mixture was then refluxed at 65–70 °C for 2 h. Finally, the condenser was replaced with a distillation apparatus, 10 mL of ethanol was added and the mixture heated at 120 °C until all the solvents were removed. The final residue is dispersed in ethanol (30 mL) for storage.
1H NMR (300 MHz, CDCl3) δ 3.92–3.81 (m, ≈1H), 3.59–3.43 (m, ≈2H), 3.50–3.38 (m, ≈2H), 2.74–2.56 (m, ≈2H), 2.65–2.46 (m, ≈2H), 1.81–1.53 (m, ≈4H), 1.33 (t, J = 7.1 Hz, ≈0.3 × 1H), 0.72–0.54 (m, ≈2H), 0.60–0.41 (m, ≈2H), 0.19–0.02 (m, ≈6H + 13 × 6H). 29Si NMR (99 MHz, Acetone) δ −13.62 (D1 OH), −14.18 (D1 OH), −14.98 (D1 OH), −19.19 (cycles D4), −21.93 to −22.21 (D2), −22.83 (D2).
2.2 Synthesis of silicon polymer P2 (with n and p around 0.1)
The synthesis was achieved in two steps.
2.2.1 Coupling of GLYMO with 2-mercaptoethanol
619 µL (2.8 mmol) of (3-glycidoxypropyl)trimethoxysilane, 199 µL (2.8 mmol) of 2-mercaptoethanol and 500 µL of methanol were mixed together in a 5 mL vial filled with argon. After the addition of 12.5 µL (0.1 mmol) of TMG (tetramethylguanidine), the mixture was stirred and heated 5 min at 60 °C and let cool down to RT for 30 min. The completion of reaction was checked by IR analysis (disappearance of epoxy bands around 900 cm−1).
2.2.2 Synthesis of polymer P2
500 µL (2.8 mmol) of (3-mercaptopropyl)-methyl-dimethoxysilane, 550 µL of MilliQ water and 50 µL of 0.1 M HCl solution were mixed together under vigorous stirring in a round bottom flask, equipped with a condenser. After 10 min, 4.1 mL (23.2 mmol) of diethoxydimethylsilane was added to the mixture. Finally, after 10 min, the modified GLYMO mixture and 380 µL (3.3 mmol) of TMG were added to the flask. The mixture was then refluxed at 120 °C for 3 h. Finally, the condenser was replaced with a distillation apparatus, until all volatiles were removed. The residue was dispersed in diethylether (20 mL), washed briefly with 10 mL of water, dried on MgSO4 and concentrated. The final viscous liquid was diluted into 20 mL of ethanol for storage.
1H NMR (300 MHz, CDCl3) δ 3.92 (m, ≈1H), 3.77 (t, J = 6.9 Hz, ≈2H), 3.45 (m, ≈4H), 2.78 (m, ≈2H), 2.68 (m, ≈2H), 2.53 (m, ≈2H), 1.64 (m, ≈4H), 1.33 (t, J = 7.1 Hz, ≈1H), 0.62 (m, ≈2H), 0.52 (m, ≈2H), 0.14–0.02 (m, ≈6H + 8x6H). 29Si NMR (99 MHz, CDCl3) δ −10.9 to 12.8 (D1 OH), −19.0 (D4 cycles), −20.7 to −23.1 (D2), −65.4 to 68.6 (T3).
2.3 Functionalization of nanoparticles
2.3.1 Quick functionalization of nanoparticles for THF extraction process
The gold nanobipyramids and nanospheres were prepared as described in our previous articles [33–36] and freshly purified by centrifugation (8000 rpm, one time) followed by redispersion in 5 mM CTAB at a concentration of 0.25 mM Au0.
Typically, 1 mL of those nanoparticles were mixed with 25 µL of an ethanolic 1 % solution of P2 (made with n = 0.08 and m = 0.1) and sonicated for 30 s in a sonic bath. 1 mL of THF was added to the mixture with manual stirring, followed by 225 µL of diethyl ether. The stirring was continued for five seconds, and then, the phase separation occurred. The water phase can be removed easily using a glass pipette. The organic phase can be diluted two times in THF for storage.
2.3.2 Functionalization of nanoparticles with isolation by centrifugation
4 mL of gold or silver nanoparticles (at 0.25 mM [Au0] or [Ag0] in 2 mM CTAB or CTAC) was mixed with 100 µL of an ethanolic 1 % solution of P2 (made with n = 0.1 and m = 0.1), sonicated for 5 min in a sonic bath. The mixture was incubated for at least one hour (up to 18 h for the bigger particles) at 50 °C. After centrifugation (8000 rpm), the particles were redispersible using a sonic bath in ethanol, acetone and THF.
3 Results and discussion
The design and synthesis of silicon-based polymers was investigated in order to (1) stabilize the gold nanoparticles in wide range of solvents and (2) overcome the instability of gold nanoparticles in sol–gel media during the formation of monolithic transparent metal doped materials.
Typically, transparent silica-based composite materials are prepared using MTEOS-based sols diluted in tetrahydrofuran (THF) [37–40]. For the present study, the selected nanoparticles possess different geometries, either anisotropic in the case of gold bipyramids or isotropic gold nanospheres. Both systems are prepared in cationic surfactants media, which make them difficult to stabilize efficiently in organic solvents such as THF. One efficient way to stabilize the AuNPs in the sol–gel media is to coat the surface of the particles with a silica shell. Unfortunately the synthesis is not always easily controlled (silica layer homogeneity) and time consuming, and the particles shape can be affected during the coating process [30, 41]. We decided to develop a new series of silicon-based polymer, which will bring strong affinity of the covered particles for the sol–gel media, and bearing thiol pending groups ensuring efficient surface grafting on the metal.
The preparation of linear functional silicon polymers can be achieved by simply mixing different alkoxysilanes monomers that can be selectively hydrolyzed together or separately in order to obtain the desired oligomers. It is important to note that for this study, the polymer length did not need to be perfectly controlled in order to improve the particle stabilization.
Since gold particles are prepared in water-based solutions, the polymers have to be compatible with such environment for efficient grafting. The first attempts to functionalize AuNPs in water with 3-mercaptopropylsilanes and diethoxydimethylsilane (DEDMS)-based polymers were unsuccessful. The main reason was because of the bad compatibility between the polymer and the water dispersion of AuNP. Therefore, an epoxy-silane, (3-glycidoxypropyl)methyldimethoxysilane (GLYDMO), was added to increase the hydrophilicity of the polymer and consequently improve the homogeneous mixing. The preparation of polymer P1 is shown in scheme 1. It was prepared and used for particle functionalization such as previously reported [40]. Unfortunately, despite its good compatibility with the particles solution, P1 led to dispersion showing low stability, in particular in THF, after few days of storage. Moreover, the reactivity toward the metal surface was sometimes too low (especially for gold nanospheres).
We assumed that to overcome this lack of reactivity and to improve the time stability, higher amount of thiol units were needed together with hydroxyl functions along the polymer chain. Indeed, coupling between the mercaptopropyl units and the epoxy occurred during the reaction leading to a lower amount of available thiols for anchoring on the AuNPs. We thus designed a system derived from P1, with several important changes. Prior to its inclusion within the polymer, the epoxy-silane was reacted with 2-thioethanol to avoid coupling on the MPDMS during the synthesis. Using this approach, more thiols were available from the mercaptopropyl units, the hydrophilicity was high and the beta-hydroxysulfide groups were still present. The GLYDMO precursor was replaced by (3-glycidoxypropyl)trimethoxysilane (GLYMO) in order to compensate the loss of epoxy/thiol crosslinking. The (3-mercaptopropyl)-methyl-dimethoxysilane (MPDMS) was hydrolyzed alone, to favor the formation of oligo-mercaptopropylsilicone blocks inside the final polymer chains. Finally, TEA was replaced with the way more basic tetramethylguanidine (TMG). Less water was used, which made the control of the chain length easier. The polymer P2 was thus obtained in two steps (Scheme 2). This polymer showed much higher reactivity toward AuNPs, which reduced the time of functionalization and increased considerably the stability of the dispersions in the final media.
The reactivity and stabilizing efficiency of the new P2 polymer was evaluated on several kinds of metallic nanoparticles exhibiting different geometries such as gold bipyramids, silver nanorods and gold nanospheres. Efficient grafting was evidenced on anisotropic particles by electronic microscopy. Figure 1 shows the polymer shell thickness covering gold bipyramids of different length. In the case of silver nanorods, aggregation was observed with non-grafted systems. Functionalized rods did not exhibit any packing (Fig. 2).
Left Transmission electron microscopy (TEM) photograph of gold nanobipyramids freshly functionalized with P2. 1 mM of NiNO3 was introduced in the particle solution to enhance polymer contrast. The particles were in contact with the polymer for one night before isolation by centrifugation. Right Scanning electron microscopy (SEM) imaging of 6-month old water dispersion of functionalized nanobipyramids showing the important growth of the thick shell
Interestingly, condensation between silanol groups along P2 could increase both the polymer chain length and densification of the shell surrounding the nanoparticles. As a direct consequence, the shell thickness could be controlled by varying the reaction time between the nanoparticles and the polymer. Figure 1 shows the impact of the reaction time on the thickness of the final shell.
Efficiency of grafting was also evidenced through solvent extraction of the particles from water to THF (Fig. 3). The metallic colloids in a surfactant/water mixture were incubated with either P1 or P2 for different durations. THF was then added, followed by a small amount of diethyl ether leading to two different phases. The organic phase was quickly separated from water and became the same color as the previous particles suspension. Moreover, the surfactant/water mixture became colorless proving that all the polymer-functionalized particles were extracted from the aqueous phase to the organic one (Fig. 3). The higher reactivity of P2 compared to P1 was evidenced by the faster incubation time from few minutes to few hours, respectively.
The THF phase was easily recovered, concentrated by evaporation and stored. Such suspension could be used directly to prepare AuNPs containing sol–gel materials (Fig. 4) [40].
Example of dispersion of 45 nm gold nanospheres (top left, respective concentrations from left to right 0.03, 0.06, 0.13, 0.25, 0.5 mM in Au0) and gold nanobipyramids (top right, respective concentrations from left to right 0.06, 0.13, 0.25 mM in Au0) at various concentrations in sol–gel silica-based materials. Side view of the materials with gold nanospheres (bottom). The diameter of the prepared xerogels is 1 cm
The ratio between the different monomers during polymer synthesis is a way to control the reactivity and the compatibility of the polymer with the organic solvent. The amount of opened epoxy units (n) mostly control the polarity and the reticulation of the polymer. For the THF extraction, polymers with a low polarity (with 0.05 < n<0.08 and p = 0.1) are better, despite being less soluble in water. When the particles are redispersed in ethanol, after centrifugation, a polymer with n = p = 0.1 gives better results. Therefore, one can tune easily the behavior and the solvent compatibility of the final particles by changing the amount of polar crosslinking units in the polymer backbone.
The spectroscopic characterization on the modified nanoparticles showed no broadening, but only a little shift of the absorption band when transferred from water to THF, in particular for anisotropic bipyramids for which the spectrum is mostly affected on the longitudinal resonance (Fig. 5). The most important shift is observed in the case of the more elongated bipyramids. Such behavior, attributed to the sharpness of the tips, was already described in previous work [33]. Similarly, when transferred into the sol–gel silica materials, no broadening of the absorption band was observed demonstrating the quality and homogeneity of the dispersion in the solid phase [40].
4 Conclusion
As a conclusion, we were able to design a new silicon-based polymer, which allowed easy and efficient surface functionalization of metal nanoparticles of different size and shape. This surface modification allowed important stabilization of the nanoobject in suspension and furthermore permitted their transfer in organic solvents such as THF for further use. Such approach is extremely convenient for the preparation of very thin layer of silicone at the surface of any metal nanoobject and even allows a good control of this thickness together with easy processing. As demonstrated by our group, this strategy gave the opportunity to prepared for instance sol–gel-based monoliths or films with incorporation of metallic nanostructures of controlled shape and size with highly homogeneous dispersion. This opens the route toward new plasmonic hybrid materials for fundamental investigations on the understanding of charge and energy transfers and further devices for enhancement of optical responses in sensors, optical filters or imaging technologies. By changing the nature of the monomers, for example using aminosilanes, the polymer was also adapted to other systems such as rare-earth fluoride or oxide nanoparticles.
References
Qu S, Gao Y, Jiang X, Zeng H, Song Y, Qiu J, Zhu C, Hirao K (2003) Nonlinear absorption and optical limiting in gold-precipitated glasses induced by a femtosecond laser. Opt Commun 224(4–6):321–327. doi:10.1016/s0030-4018(03)01761-9
Qu SL, Zhao CJ, Jiang XW, Fang GY, Gao YC, Zeng HD, Song YL, Qui JR, Zhu CS, Hirao K (2003) Optical nonlinearities of space selectively precipitated Au nanoparticles inside glasses. Chem Phys Lett 368(3–4):352–358. doi:10.1016/S0009-2614(02)01885-7
Porel S, Venkatram N, Rao DN, Radhakrishnan TP (2007) Optical power limiting in the femtosecond regime by silver nanoparticle-embedded polymer film. J Appl Phys 102(3):033107. doi:10.1063/1.2764239
Porel S, Venkatram N, Rao DN, Radhakrishnan TP (2007) In situ synthesis of metal nanoparticles in polymer matrix and their optical limiting applications. J Nanosci Nanotechnol 7(6):1887–1892. doi:10.1166/jnn.2007.736
Mangelson BF, Jones MR, Park DJ, Shade CM, Schatz GC, Mirkin CA (2014) Synthesis and characterization of a plasmonic-semiconductor composite containing rationally designed, optically tunable gold nanorod dimers and anatase TiO2. Chem Mater 26(12):3818–3824. doi:10.1021/cm5014625
Wang D, Zhou ZH, Yang H, Shen KB, Huang Y, Shen S (2012) Preparation of TiO2 loaded with crystalline nano Ag by a one-step low-temperature hydrothermal method. J Mater Chem 22(32):16306–16311. doi:10.1039/c2jm16217b
Besson S, Gacoin T, Ricolleau C, Boilot JP (2003) Silver nanoparticle growth in 3D-hexagonal mesoporous silica films. Chem Commun 3:360–361. doi:10.1039/b208357d
Gacoin T, Besson S, Boilot JP (2006) Organized mesoporous silica films as templates for the elaboration of organized nanoparticle networks. J Phys Condens Matter 18(13):S85–S95. doi:10.1088/0953-8984/18/13/S06
Pu-Wei Wu WC, Martini Ignacio B, Dunn Bruce, Schwartz Benjamin J, Yablonovitch Eli (2000) Two-photon photographic production of three-dimensional metallic structures within a dielectric matrix. Adv Mater 12(19):1438–1441
Battie Y, Destouches N, Bois L, Chassagneux F, Moncoffre N, Toulhoat N, Jamon D, Ouerdane Y, Parola S, Boukenter A (2009) Generation of an ordered layer of silver nanoparticles in mesostructured dielectric films. J Nanopart Res 12(3):1073–1082. doi:10.1007/s11051-009-9794-8
Bois L, Bessueille F, Chassagneux F, Battie Y, Destouches N, Hubert C, Boukenter A, Parola S (2008) Silver nanoparticles growth in a mesoporous silica film templated with the F127 triblock copolymer. Colloids Surf A 325(1–2):86–92. doi:10.1016/j.colsurfa.2008.04.045
Bois L, Chassagneux F, Battie Y, Bessueille F, Mollet L, Parola S, Destouches N, Toulhoat N, Moncoffre N (2010) Chemical growth and photochromism of silver nanoparticles into a mesoporous titania template. Langmuir 26(2):1199–1206. doi:10.1021/la902339j
Bois L, Chassagneux F, Desroches C, Battie Y, Destouches N, Gilon N, Parola S, Stephan O (2010) Electroless growth of silver nanoparticles into mesostructured silica block copolymer films. Langmuir 26(11):8729–8736. doi:10.1021/la904491v
Bois L, Chassagneux F, Parola S, Bessueille F, Battie Y, Destouches N, Boukenter A, Moncoffre N, Toulhoat N (2009) Growth of ordered silver nanoparticles in silica film mesostructured with a triblock copolymer PEO–PPO–PEO. J Solid State Chem 182(7):1700–1707. doi:10.1016/j.jssc.2009.01.044
De S, De G (2008) In situ generation of Au nanoparticles in UV-curable refractive index controlled SiO2–TiO2–PEO hybrid films. J Phys Chem C 112:10378–10384
Ferrara MC, Mirenghi L, Mevoli A, Tapfer L (2008) Synthesis and characterization of sol–gel silica films doped with size-selected gold nanoparticles. Nanotechnology 19(36):365706. doi:10.1088/0957-4484/19/36/365706
Pal S, De G (2008) Formation of Au–Pt bimetallic nanoparticles in a two-layer SiO2 films doped with Au and Pt, respectively, through interlayer diffusion. Phys Chem Chem Phys 10(27):4062–4066. doi:10.1039/b803052a
Martinez ED, Boissiere C, Grosso D, Sanchez C, Troiani H, Soler-Illia GJAA (2014) Confinement-induced growth of au nanoparticles entrapped in mesoporous TiO2 thin films evidenced by in situ thermo-ellipsometry. J Phys Chem C 118(24):13137–13151. doi:10.1021/jp500429b
Formanek F, Takeyasu N, Tanaka T, Chiyoda K, Ishikawa A, Kawata S (2006) Three-dimensional fabrication of metallic nanostructures over large areas by two-photon polymerization. Opt Express 14(2):800–809
Kaneko K, Sun H-B, Duan X-M, Kawata S (2003) Two-photon photoreduction of metallic nanoparticle gratings in a polymer matrix. Appl Phys Lett 83(7):1426. doi:10.1063/1.1601302
Kalfagiannis N, Karagiannidis PG, Pitsalidis C, Hastas N, Panagiotopoulos NT, Patsalas P, Logothetidis S (2014) Performance of hybrid buffer poly(3,4-ethylenedioxythiophene) poly(styrenesulfonate) layers doped with plasmonic silver nanoparticles. Thin Solid Films 560:27–33. doi:10.1016/j.tsf.2014.01.032
Stellacci F, Bauer CA, Meyer-Friedrichsen T, Wenseleers W, Alain V, Kuebler SM, Pond SJK, Zhang Y, Marder SR, Perry JW (2002) Laser and electron-beam induced growth of nanoparticles for 2D and 3D metal patterning. Adv Mater 14(3):194–198
Kinnear C, Dietsch H, Clift MJD, Endes C, Rothen-Rutishauser B, Petri-Fink A (2013) Gold nanorods: controlling their surface chemistry and complete detoxification by a two-step place exchange. Angew Chem Int Ed 52:1934–1938
Zhang Z, Lin M (2014) Fast loading of PEG-SH on CTAB-protected gold nanorods. RSC Adv 4:17760–17767
Li J, Zhu B, Zhu Z, Zhang Y, Yao X, Tu S, Liu R, Jia S, Yang CJ (2015) Simple and rapid functionalization of gold nanorods with oligonucleotides using an mPEG-SH/Tween 20-assisted approach. Langmuir 31(28):7869–7876
Thierry B, Ng J, Krieg T, Griesser HJ (2009) A robust procedure for the functionalization of gold nanorods and noble metal nanoparticles. Chem Commun 1(13):1724–1726
Alkilany AM, Thompson LB, Murphy CJ (2010) Polyelectrolyte coating provides a facile route to suspend gold nanorods in polar organic solvents and hydrophobic polymers. ACS Appl Mater Interfaces 2(12):3417–3421
Gole A, Murphy CJ (2005) Polyelectrolyte-coated gold nanorods: synthesis, characterization and immobilization. Chem Mater 17(6):1325–1330. doi:10.1021/cm048297d
Sivapalan ST, Vella JH, Yang TK, Dalton MJ, Swiger RN, Haley JE, Cooper TM, Urbas AM, Tan LS, Murphy CJ (2012) Plasmonic enhancement of the two photon absorption cross section of an organic chromophore using polyelectrolyte-coated gold nanorods. Langmuir 28(24):9147–9154. doi:10.1021/la300762k
Pastoriza-Santos I, Pérez-Juste J, Liz-Marzán LM (2006) Silica-coating and hydrophobation of CTAB-stabilized gold nanorods. Chem Mater 18:2465–2467
Yang J, Wu J, Wu Y, Wang J, Chen C (2005) Organic solvent dependence of plasma resonance of gold nanorods: a simple relationship. Chem Phys Lett 416:215–219
Mitamura K, Imae T, Saito N, Takai O (2007) Fabrication and self-assembly of hydrophobic gold nanorods. J Phys Chem B 111(30):8891–8898
Chateau D, Liotta A, Vadcard F, Navarro JR, Chaput F, Lerme J, Lerouge F, Parola S (2015) From gold nanobipyramids to nanojavelins for a precise tuning of the plasmon resonance to the infrared wavelengths: experimental and theoretical aspects. Nanoscale 7:1934–1943. doi:10.1039/c4nr06323f
Navarro JR, Lerouge F, Micouin G, Cepraga C, Favier A, Charreyre MT, Blanchard NP, Lerme J, Chaput F, Focsan M, Kamada K, Baldeck PL, Parola S (2014) Plasmonic bipyramids for fluorescence enhancement and protection against photobleaching. Nanoscale 6:5138–5145. doi:10.1039/c3nr06425e
Navarro JR, Manchon D, Lerouge F, Cottancin E, Lerme J, Bonnet C, Chaput F, Mosset A, Pellarin M, Parola S (2012) Synthesis, electron tomography and single-particle optical response of twisted gold nano-bipyramids. Nanotechnology 23:145707. doi:10.1088/0957-4484/23/14/145707
Navarro JR, Manchon D, Lerouge F, Blanchard NP, Marotte S, Leverrier Y, Marvel J, Chaput F, Micouin G, Gabudean AM, Mosset A, Cottancin E, Baldeck PL, Kamada K, Parola S (2012) Synthesis of PEGylated gold nanostars and bipyramids for intracellular uptake. Nanotechnology 23:465602. doi:10.1088/0957-4484/23/46/465602
Zieba R, Desroches C, Chaput F, Carlsson M, Eliasson B, Lopes C, Lindgren M, Parola S (2009) Preparation of functional hybrid glass material from platinum (II) complexes for broadband nonlinear absorption of light. Adv Func Mater 19(2):235–241. doi:10.1002/adfm.200801008
Chateau D, Chaput F, Lopes C, Lindgren M, Brannlund C, Ohgren J, Djourelov N, Nedelec P, Desroches C, Eliasson B, Kindahl T, Lerouge F, Andraud C, Parola S (2012) Silica hybrid sol–gel materials with unusually high concentration of Pt-organic molecular guests: studies of luminescence and nonlinear absorption of light. ACS Appl Mater Interfaces 4(5):2369–2377. doi:10.1021/am2015537
Château D, Bellier Q, Chaput F, Feneyrou P, Berginc G, Maury O, Andraud C, Parola S (2014) Efficient hybrid materials for optical power limiting at telecommunication wavelengths. J Mater Chem C 2(26):5105. doi:10.1039/c4tc00193a
Lundén H, Liotta A, Chateau D, Lerouge F, Chaput F, Parola S, Brännlund C, Ghadyani Z, Kildemo M, Lindgren M, Lopes C (2015) Dispersion and self-orientation of gold nanoparticles in sol–gel hybrid silica–optical transmission properties. J Mater Chem C 3(5):1026–1034. doi:10.1039/c4tc02353f
Abadeer NS, Brennan MR, Wilson WL, Murphy CJ (2014) Distance and plasmon wavelength dependent fluorescence of molecules bound to silica-coated gold nanorods. ACS Nano 8(8):8392–8406
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chateau, D., Liotta, A., Gregori, D. et al. Controlled surface modification of gold nanostructures with functionalized silicon polymers. J Sol-Gel Sci Technol 81, 147–153 (2017). https://doi.org/10.1007/s10971-016-4116-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-016-4116-y