Abstract
Indium tin oxide (ITO) is recognized as the best transparent and conductive material [transparent conducting oxide (TCO)] until now and its properties are dependent on the preparation method. In the present work ITO films with In:Sn atomic ratio 9:1 were prepared by a sol–gel route on different substrates (microscope glass slides, microscope glass covered with one layer of SiO2 and Si wafers) for TCO applications. The multilayer ITO films were obtained by successive deposition by the dip-coating method and the films were characterized from the structural, morphological, optical, and electrical points of view using X-ray diffraction, scanning electron microscopy, atomic force microscopy, spectroscopic ellipsometry and by Hall effect measurements, respectively. The results showed that the thickness, optical constants and carrier numbers depend strongly on the type of substrate, number of deposited layers and sol concentration. The optical properties of ITO films are closely related to their electrical properties. The enhancement of the conductivity was possible with the increase of crystallite size (which occurred after thermal treatment) and with the reduction of surface roughness.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Indium tin oxide (ITO) is an important material as it is one of the most widely used transparent conducting oxides (TCOs) required as a component in photovoltaic cells [1, 2], electroluminescent devices [3], transparent electrodes in plasma display panels [4], sensor modules [5], protective coatings [6] and so on.
Properties required for TCOs are low resistivity (as 1.0 × 10−4 Ω cm), conductivities higher than 103 S/cm, low extinction coefficient k in the optical visible (Vis) spectral range, wide band gap (Eg > 3.0 eV) that ensure the transparence in visible range and optical transmittance higher than 80 %.
In order to ensure the required properties of the transparent conductive oxides, several methods of preparation were used, such as: sputtering [7–15], spray pyrolysis [16–18], screen printing [19, 20], ion beam assisted deposition [21, 22], microwave [23], evaporation [15, 24, 25] and sol–gel [26–33]. More recently, ITO nanocrystals have been synthesized by hydrothermal process followed by annealing at 450 °C in forming gas for 1 h [34]. The post densification reducing thermal treatment in forming gas was performed to decrease the resistivity of the ITO films by a factor of two [19, 34].
The sol–gel method presents some interesting advantages over other methods of coatings deposition, such as: easy control of the final materials, the possibility of deposition on complex shaped substrates, easy control of doping concentration, structural homogeneity, low temperature of thermal treatment of films, as well as the low cost equipments.
In the present work indium thin oxide transparent films with In:Sn atomic ratio of 9:1 were deposited by a sol–gel route on several substrates: microscope glass, microscope glass covered with one layer of SiO2 (prepared also by sol–gel method) and Si wafers.
The interest to study the influence of the substrate on the ITO films properties is important for their application as solar cells but also for their newer applications as sensors or protective coatings [5, 6].
The ITO films obtained by sol–gel method were characterized from the morphological, optical, and electrical points of view and the results showed that all physical properties depend strongly on the type of the substrate, solution concentration and number of layers.
2 Experimental
2.1 Solution preparation and film deposition
ITO films with In:Sn atomic ratio 9:1 were prepared as follows: indium nitrate was used as In2O3 source, 2 tin ethylhexanoate as SnO2 source and 2,4-pentanedione as chelating agent, in ethanol solvent.
The composition of the initial solution and the experimental conditions for obtaining sol–gel ITO films are based on the previous published results [27, 29]. Solutions with 0.1 and 0.25 M concentrations were investigated (denoted from here on as 0.1 and 0.25 M ITO). The following molar ratio of the reagents were used: In:Sn = 9:1 and In:EtOH:2,4-pentanedione = 1:35:0.05 for 0.1 M and In:EtOH:2,4-pentanedione = 1:14:0.05 for 0.25 M solutions.
-
1.
Before deposition the viscosity of the solutions was measured with the Brookfield Viscometer DV-II + Pro equipment at room temperature in 8 ml of solution. As expected, the viscosities of solutions show higher values when their concentration is higher (1.37 for the 0.1 M ITO sol and 1.47 for the 0.25 M ITO sol).
-
2.
From the solution prepared as presented above, films were deposited on glass, glass covered with SiO2 layer (prepared by sol–gel) and Si wafer substrate by dip-coating method with a withdrawal rate of 5 cm/min. Before deposition, the solutions were aged for 24 h. For multiple depositions, after each deposition an intermediary thermal treatment of densification at 260 °C for 10 min, using a heating rate of 5 °C/min, was used. After the last deposition the films were thermally treated at 400 °C for 2 h in air, using a heating rate of 5 °C/min.
2.2 Film characterization
The crystallinity of the sol–gel ITO films was studied by X-ray diffraction (XRD) method. The measurements were performed using Rigaku Ultima IV equipment, with Cu Kα radiation and a fixed power source (40 kV and 30 mA). The diffractometer was set in condition of grazing incident X-ray diffraction (GIXRD) with ω = 0.5°. The mean crystallite size was evaluated from the line broadening of (222) XRD line of ITO thin films using Scherrer’s equation: D = 0.94λ/βcosθ, where λ = 1.54 Å is the X-ray (CuKα) wavelength, θ the diffraction angle, β the full width at half maximum of the (222) diffraction line and 0.94 is the Scherrer constant.
Systematic information on the ITO films morphology and thickness was obtained by scanning electron microscopy (SEM) analysis using a FEI Quanta 3D microscope operating at 5 kV.
Atomic force microscopy (AFM) measurements were carried in the non-contact mode, with a XE-100 apparatus from Park Systems, using sharp tips (highly doped-Si material, <8 nm tip radius; PPP-NCHR type from Nanosensors™). The topographical 2D and 3D AFM images were taken over the area of 2 × 2 μm2; for displaying purpose and subsequent statistical data analysis [including the calculation of the root mean square (RMS) roughness] XEI (v.1.8.0) Image Processing Program developed by Park Systems was used.
Ellipsometric measurements were performed in order to obtain the thickness and optical (dielectric) constants with J.A. Woollam Co., Inc equipment composed of a rotating-analyzer VASE ellipsometer for UV–VIS–NIR range. Measurements have been performed at room temperature, using 70° as incidence angle with 10 nm wavelength step. The film thickness and the refractive index (n) were obtained from the ellipsometric data analysis with an accuracy of ±0.2 nm and ±0.005 respectively. The optical transmission measurements were performed at 0° incidence angle on the same apparatus.
Electrical measurements based on Hall effect, were performed on a HMS-5000 equipment from Ecopia using van der Pauw method.
3 Results and discussion
In the experimental conditions presented above, films up to ten layers could be obtained using the solution with 0.1 M concentration, while only five layers could be deposited in the case of the solution with 0.25 M concentration, because it gellified due to its higher viscosity.
The different substrates used for films deposition were selected taking into account the application of the considered films, such as: solar cell [1–4] sensors [5] or protective coatings [6].
The SEM images of the films obtained from 0.1 to 0.25 M sol concentrations and after ten and five layers, respectively, are presented in Figs. 1 and 2 in comparison with AFM images at the same scale. The sol concentration has an important effect on the film microstructure: the higher viscosity leads to coarser features, including roughness and pore size, confirmed by the AFM line profiles (Figs. 1, 2).
The thicknesses of the ITO films were determined by SEM measurements of the films seen edge-on (Fig. 3). The measured thicknesses of the 0.25 M ITO films deposited on glass, SiO2/glass and Si substrates are: 65 ± 15, 200 ± 20 and 90 ± 10 nm, respectively. The SiO2 layer deposited on glass can also be observed in the SEM micrograph in Fig. 3, with a thickness of 50 ± 5 nm. The local variations of thickness are due to the roughness of the films. The ITO film deposited on Si shows the smoothest surface and also the smallest variation in thickness along the film, while the thickest film with the roughest surface have been observed for the film deposited on the SiO2/glass substrate. The film deposited on glass is the thinnest but its roughness is close to the films deposited on SiO2/glass substrates.
Figure 4 shows SEM micrographs of the edge of two ITO films deposited on SiO2/glass substrate: 0.1 M ITO with ten layers and 0.25 M ITO with five layers. The sol concentration clearly has a strong influence on the thickness. For the 0.1 M ITO sample, the film thickness is barely distinguishable from the SiO2 film and it is not higher than ~20 nm, while it is ~200 nm for 0.25 M ITO film after five depositions. The presence of pores with several hundreds nm diameter is also observed in the 0.25 M ITO films after five depositions. The coarse porosity is observed only for films with more than two layers, on the samples prepared with sol concentration 0.25 M, especially on the glass and SiO2/glass substrates.
Atomic force microscopy measurements revealed the effect of the overall RMS roughness of layer deposition for the 0.25 M ITO films (Fig. 5), showing the effect of the formation of coarser features on the film surface. The RMS roughness increases with the number of depositions almost linearly for 1–5 layers on Si substrate and changes abruptly for SiO2/glass and glass substrate (three times larger than on Si substrate) after five layers deposition, due to the formation of wider surface cavities (in the range of hundreds nm diameter)—Fig. 5. The roughness of the bare substrates is similar for Si and SiO2/glass (even <1 nm at 2 × 2 nm2) and ~2 nm (at 2 × 2 nm2) for the glass explaining the slightly higher roughness values obtained after deposition of 1–2 layers of 0.25 M ITO on glass substrate—Fig. 5. On the other hand, the large pores formed after five layers deposition on Si are flattened (around 10 nm deep) as compared to the superficial pores formed on glass (~20–25 nm deep) or SiO2/glass (~25–30 nm deep) substrates—Fig. 2—and could explain the roughness behavior observed in Fig. 5 for multilayered 0.25 M ITO films.
The structural characterization of the nanocrystalline ITO films demonstrates that an important parameter during film synthesis is the concentration (and viscosity) of the solution. Low concentration of 0.1 M ITO results in films with similar thicknesses after ten layers deposition (Table 1) between 22 and 31 nm and reduced roughness (line profiles with vertical scale of ±2 nm, Fig. 1), for all three substrates. This shows a small effect of the substrate on the successive densification of each 0.1 M ITO layer during thermal treatments. Good wetting of the substrate by the solution leads to the formation of a homogeneous, continuous layer after the first few depositions and thermal treatments.
We can estimate, from XRD measurements (presented further), that the thicknesses of the ten layers 0.1 M ITO films approximates the size of three ITO crystallites for glass and Si substrates, and ~3.7 for SiO2/glass. The last mentioned substrate induced the thickest films because it is the most reactive one due to the presence of SiO2 sol–gel layer.
A concentration of 0.25 M ITO leads to significantly thicker films and the formation of coarse porosity. The higher viscosity of the solution hinders the formation of a continuous, homogeneous layer during the first deposition and can promote film growth in the direction perpendicular to the substrate surface, leading to the formation of thicker films and coarser pores.
We can conclude that perpendicular growth of the 0.25 M ITO films after five layers deposition depends significantly on the reactivity of the substrate with the solution: it is significantly lower-around 80 ± 15 nm—on highly stable, dense, non-reactive surfaces, such as those of glass and Si, than in the case of SiO2/glass substrate—200 ± 20 nm—(Fig. 3).
Multiple layers deposition has a high contribution in the reduction of pore size. The high resolution SEM micrographs (Fig. 6) show the effect of multiple layers deposition in the nanoscale intrinsic porosity of the 0.1 M ITO samples on SiO2/glass, after two and ten layers. There is a clear reduction of the pore size at the nanoscale. For films with two layers, pore sizes of 20–30 nm are observed, while for films with ten layers only much smaller pores are visible (between 5 and 10 nm), as it is shown in the inset of Fig. 6. The same effect of additional layers deposition on nanoscale porosity has been observed on all studied substrates. We attribute the reduction in pore size to their filling with solution by subsequent depositions and to the densification of the films during thermal treatments carried out after each deposition. The reduction in porosity seems to be associated to the observed increase of carrier concentration and lower resistivity after multiple depositions, discussed below.
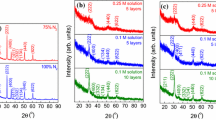
X-ray diffraction results are presented in Fig. 7 for 0.1 M ITO and Fig. 8 for 0.25 M ITO films annealed at 400 °C. They are polycrystalline and have monophasic cubic bixbyite structure [35] as can be assigned from ICDD file no. 01-089-4596. Diffraction lines are more or less intense following the crystallization degree of the sample when compared to bixbyite structure. The film exhibits the reflection from the (222) plane as the most predominant peak in the XRD pattern, but no preferred orientation can be noticed. None of the patterns present any characteristic lines of tin oxides phases, which indicates that the tetravalent Sn4+ replaces In3+ substitutionally into the indium oxide lattice, retaining the In2O3 structure. For 0.1 M ITO films deposited on all substrates the XRD patterns show a similar intensity of the diffraction lines indicating a close degree of crystallinity.
For the above-mentioned films, the substrate influences the crystallite size and the unit cell parameters of the crystal structures. The crystallite size values are 9.7 ± 3.3 nm for the films deposited on glass, 10.6 ± 0.3 nm for the films deposited on SiO2/glass, and 9.3 ± 0.3 nm for the films on Si(100). The lattice constant, a, of the cubic structure is 10.0788 ± 0.0005 Å for crystals grown on glass, 10.1068 ± 0.0003 Å for crystals grown on SiO2/glass and 10.0925 ± 0.0002 Å crystals grown on Si(100).
A vitreous material is not completely random. There are atoms organized in isolated structures where the interatomic distances are approximately constant [36]. This short distance ordering is detected as a single or few broad “diffusion lines” on the diffraction pattern. These lines correspond to the interference of atoms positioned more often at the representative distance, d [37].
On the thin films starting from 0.25 M solution, the X-rays diffraction lines have low intensities, even after five depositions and subsequent thermal treatment and only a broad line corresponding to the (222) plane of the ITO structure can be noticed. The degree of crystallinity is lower when compared to the samples starting from 0.1 M ITO solution because the 0.25 M ITO solution is leading to the formation of thick films that require a longer time to remove the solvent. The removal of the solvent retards the crystallization of the films, and therefore the ITO 0.25 M films are not well-crystallized.
The optical properties of the films were studied by spectroscopic ellipsometry in the 350–800 nm spectral range. The ellipsometric measurements were fitted with three layer model: surface roughness layer/ITO film/substrate. The roughness layer was modelled by EMA (50 % voids + 50 % ITO film) and the ITO film was fitted with a Cauchy model in the transparency region to find out the thicknesses of the film and of SiO2 layer between the substrate and film (in the cases of Si and SiO2/glass substrates).
From the best fit the thickness layers were obtained (see also Table 1). The thickness of SiO2 buffer layer, measured on a bare substrate without film was 45.6 nm, a value in good agreement with SiO2 layer measured by SEM. It can be observed that the thickness of the ITO films:
-
increases with the sol concentration of ITO. The thicknesses of the 0.25 M ITO five layers films on all three substrates are higher than that of 0.1 M ITO ten layers films. This is very important information for the conductivity measurement.
-
depends on the substrate; when SiO2 buffer layer is present the ITO films are thicker.
The deposition number has a smaller effect on ITO films thickness in the case of diluted solution (0.1 M). This can be related to the film densification that occurs as a result of the thermal annealing after each deposition. Accordingly, each additional deposition causes mainly a decrease in the porosity of the ITO film rather than a significant increase of the thickness.
The refractive index dispersia (n) for all samples were determined from the best ellipsometric fit (Fig. 9).
For both substrates and solution concentrations refractive index increases with the number of layers and depends on the ITO molar concentration and substrate. All samples exhibit a very weak absorption (the extinction coefficient is practically zero) from visible to near-IR range.
With increasing of refractive index, reflection losses increases and transparency decreases. For transparent films with refractive indices varying between ~1.6 and 2.2, the reflection losses at normal incidence on the films is of the order of ~5–14 %, so the transmission lowers from 90 to 76 %. For solar cell application we tried to obtain films with high transmission values, thus means small refractive index. To this end the sol–gel method is a suitable one, because we can model the proprieties of the films (e.g., refractive index) changing the ITO molar concentration, number of layers or the substrate type. Moreover the sol–gel method is known to produce films with lower refractive indices than other methods due to the intrinsic porosity of the films associated with this technique.
The transmission of ITO sol–gel films was measured by ellipsometry in the range of 250–800 nm (Fig. 10) and it was found that all values are over 90 % for one layer films on both substrates (glass and SiO2/glass) and decrease until 80 % for ten layers 0.1 M ITO films, respectively five layers 0.25 M ITO films, due to the reasons discussed above.
On the other hand a good conductivity can not be obtained for films with low number of layers, because it is not possible to obtain a high enough carrier concentration (ND) and low enough resistivity (ρ). We attribute this effect to the high porosity of the films obtained after only 1–2 layers deposition. Thus we have focused on the study of the electrical properties for samples with ten (0.1 M ITO) and five (0.25 M ITO) layers (see Table 1). The concentration of the sol from which the samples are deposited has also significant influence. Lower sol concentration leads to the diminishing of the resistivity, independent of the substrate type. The most promising results were obtained for films with ten layers obtained from solution of 0.1 M concentration, deposited on glass and SiO2/glass. These samples exhibited high carrier concentration and relatively low resistivity, but also a high recombination rate. It is very clear that the number of layers plays a crucial role both in transmission and in the electrical parameters.
Hall measurements were carried out for 0.1 M ITO sol–gel films with ten layers. The samples that gave the most promising results at room temperature (concentration 0.1 M, ten layers, glass and SiO2 covered glass) were also measured at variable temperature between 110 and 350 K—Fig. 11.
Both samples exhibited typical semiconductor behavior where resistivity decreased with increasing temperature. For the sample deposited on SiO2 covered glass substrate, carrier concentration was generally flat over the temperature range used. There is also evidence of background noise especially at lower temperatures. The sample deposited on glass registers a slight increase in both carrier concentration and mobility with temperature.
A decrease in resistivity takes place between 280 and 300 K, dominated by the sudden increase in carrier concentration. At high temperature, the thermally generated intrinsic carriers outnumber the dopants and carrier concentration increases with temperature.
The larger crystallites may enhance the conductivity of ITO films due to fewer grain boundaries [38].
4 Conclusions
Continuous and homogeneous films were deposited on three different substrates (glass, SiO2/glass and silicon) by dip-coating method from of 0.1 and 0.25 M sol concentrations. The influence of solution concentrations, number of layers and type of substrate on the structural, morphological, optical and electrical properties of the ITO films was studied by XRD, SEM, AFM, SE, optical transmission and electrical measurements. ITO structure was found on all studied films, but with different degrees of crystallinity, function of the number of depositions (which in its turn is correlated with the number of thermal treatments) and sol concentration. The smoothest surface with the smallest variation in thickness along the film was observed on Si substrate, while the SiO2/glass substrate results in the roughest film surface. As general remark, the ITO films obtained from 0.1 M solution with ten layers deposited on glass and SiO2/glass present the best TCO characteristics, namely transmission around 90 % and the carrier concentration around 1020 cm−3. The results of these experiments demonstrated the possibility to select a suitable substrate, sol-concentration and number of layers deposition for a certain application.
References
Lunt RR, Bulovic V (2011) Appl Phys Lett 98:113305
Tien WC, Chu AK (2014) Sol Energ Mater Sol C 120:18–22
Furukawa K, Terasaka Y, Ueda H, Matsumura M (1997) Synth Met 91(1–3):99–101
Li Z-H, Cho E-S, Kwon SJ (2010) Appl Surf Sci 257(3):776–780
Celik E, Aybarc U, Ebeoglugil MF, Birlik I, Culha O (2009) J Sol–Gel Sci Technol 50:337–347
Sierros KA, Morris NJ, Kukureka SN, Cairns DR (2009) Wear 267(1–4):625–631
Ahn MH, Cho E-S, Kwon SJ (2011) Appl Surf Sci 258:1242–1248
Houng B, Lin SL, Chen SW, Wang A (2011) Ceram Int 37:3397–3403
Lee CJ, Lin HK, Li CH, Chen LX, Lee CC, Wu CW, Huang JC (2012) Thin Solid Films 522:330–335
Manavizadeh N, Boroumand FA, Asl-Soleimani E, Raissi F, Bagherzadeh S, Khodayari A, Rasouli MA (2009) Thin Solid Films 517:2324–2327
Song S, Yang T, Liu J, Xin Y, Li Y, Han S (2011) Appl Surf Sci 257:7061–7064
Stroescu H, Anastasescu M, Preda S, Nicolescu M, Stoica M, Stefan N, Kampylafka V, Aperathitis E, Modreanu M, Zaharescu M, Gartner M (2013) Thin Solid Films 541:121–126
Wasa K, Hayakawa S (1991) Handbook of sputter deposition technology. Noyes Publications, NJ
Facchetti A, Marks TJ (2010) Transparent electronics: from synthesis to applications. Wiley, New York
Ginley DS, Hosono H, Paine DC (2010) Handbook of transparent conductors. Springer, New York
Rozati SM, Ganj T (2004) Renew Energy 29(10):1671–1676
El Rhaleb H, Benamar E, Rami M, Roger JP, Hakam A, Ennaoui A (2002) Appl Surf Sci 201(1–4):138–145
Bisht H, Eun H-T, Mehrtens A, Aegerter MA (1999) Thin Solid Films 351:109–114
Madhi I, Saadoun M, Bessais B (2012) Procedia Eng 47:192–195
Mbarek H, Saadoun M, Bessais B (2006) Mater Sci Eng C 26(2–3):500–504
Meng L-J, Gao J, Silva RA, Song S (2008) Thin Solid Films 516:5454–5459
Zhinong Y, Yuqiong L, Fan X, Zhiwei Z, Wei X (2009) Thin Solid Films 517:5395–5398
Okuya M, Ito N, Shiozaki K (2007) Thin Solid Films 515:8656–8659
Fallah HR, Varnamkhasti MG, Vahid MJ (2010) Renew Energy 35:1527–1530
Senthilkumar V, Vickraman P, Jayachandran M, Sanjeeviraja C (2010) Vacuum 84:864–869
Stoica TF, Stoica TA, Vanca V, Lakatos E, Zaharescu M (1999) Thin Solid Films 348:273–278
Stoica TF, Stoica TA, Zaharescu M, Popescu M, Sava F, Popescu-Pogrion N, Frunză L (2000) J Optoelectron Adv Mater 2:684–688
Alam MJ, Cameron DC (2000) Thin Solid Films 377:455–459
Stoica TF, Gartner M, Losurdo M, Teodorescu VS, Blanchin M, Stoica T, Zaharescu M (2004) Thin Solid Films 455–456:509–512
Biswas PK, De A, Dua LK, Chkoda L (2006) Appl Surf Sci 253:1953–1959
Beaurain A, Luxembourg D, Dufour C, Koncar V, Capoen B, Bouazaoui M (2008) Thin Solid Films 516:4102–4106
Valencia HY, Moreno LC, Ardila AM (2008) Microelectron J 39:1356–1357
Prodi-Schwab A, Luthe Th, Jahn R, Herbig B, Lobmann P (2008) J Sol–Gel Sci Technol 47:68–73
Soliemana A, Zayeda MK, Alamria SN, Al-Dahoudib N, Aegerter MA (2012) Mater Chem Phys 134:127–132
Liu J, Wu D, Zhang N, Wang Y (2010) Rare Met 29(2):143–148
Kittel C (1996) Introduction to solid state physics. Wiley, New York
Van Meerssche M, Feneau-Dupont J (1984) Introduction à la cristallographie et à la chimie structurale. Editions Peeters, Leuven
Su C, Sheu TK, Chang YT, Wan MA, Feng MC, Hung WC (2005) Synth Met 153:9–12
Acknowledgments
This work was supported by the Romanian PN-II-ID-PCE-2011-3-0446 Project and by EU (ERDF) and Romanian Government that allowed for acquisition of the research infrastructure under POS-CCE O 2.2.1 Project INFRANANOCHEM—Nr. 19/01.03.2009.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Predoana, L., Preda, S., Nicolescu, M. et al. Influence of the substrate type on the microstructural, optical and electrical properties of sol–gel ITO films. J Sol-Gel Sci Technol 71, 303–312 (2014). https://doi.org/10.1007/s10971-014-3373-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-014-3373-x