Abstract
Nitrogen doped TiO2 (N-TiO2) nanoparticles with about 30 nm in size were produced by a sol–gel method and characterized respectively by UV–vis, X-ray diffraction (XRD), Transmission electron microscopy, X-ray photoelectron spectroscopy (XPS). Their photocatalytic antibacterial properties were evaluated by the antibacterial ratio against Escherichia coli in dark and under simulated sunlight respectively. The XRD pattern showed that the doped nano-TiO2 was mainly composed of anatase phase. The XPS spectra of the N-TiO2 sample indicated that TiO2 was doped by nitrogen atom. The nitrogen doping created a new N 2p state slightly above the valence band top consists of O 2p state, and this pushes up the valence band top and decreased the band gap. Which leaded to the absorption edge was red-shifted to the visible light region of UV–vis spectra of nitrogen doped nano-TiO2 comparing with pure nano-TiO2. The antibacterial percentage of N-TiO2 against E. coli reached to 90 % under simulated sunlight for 2 h, which was much better than that in dark, also than that of pure nano-TiO2. The photo-catalytic antibacterial activity was activated under visible light. The structure and integrity of cell wall and cell membrane were destructed, and even caused the bacteria death.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
A new avenue for water sterilization has been created, since Matsunaga and co-workers [1] reported that microbial cells in water could be killed by contact with a TiO2-Pt photocatalyst upon irradiation with UV light for 60–120 min in 1985. TiO2-mediated disinfection seems to be a promising technique compared to the common disinfection methods such as chlorination and UV disinfection. For the shortcoming of the methods and the emergence of more resilient and virulent strains of microorganisms, it is need for more effective sterilization technologies and antibacterial materials.
The primary event occurring on the illuminated TiO2 is the generation of e −cb (photogenerated electrons) and h +vb (photogenerated holes). In these reactions, the organic matter are oxidized by the photogenerated holes or by reactive oxygen species such as ·OH and O2 − radicals formed on the irradiated TiO2 surface [2]. However, it is a problem in the application of TiO2 as a photocatalyst that the band gap energy of TiO2 is too large, i.e. the band gap value of anatase is 3.2 eV so that it only shows photocatalytic activity under near-UV region(λ ≤ 390 nm), which is about 3 % of the solar spectrum. Due to this inherent limitation, solar energy cannot be utilized efficiently for photocatalytic disinfection.
To improve the efficiency, a new approach to broaden the photoresponse of TiO2 by doping with a nonmetal atom has been introduced. Recently, some groups have reported that nonmetal doped into TiO2 such as boron [3], carbon [4], nitrogen [5], fluorine [6], sulfur [7, 8], chlorine and bromine [9], iodine [10] to achieve enhanced visible light photocatalytic activities undeniably, doping TiO2 photocatalyst with a nonmetal element becomes a hot research topic, and it opens up new possibilities for the development of solar-induced photocatalytic materials.
Asahi et al. [11] reported theoretical calculations of the band structure of nitrogen-doped TiO2 and its visible light photocatalytic degradation of acetaldehyde and methylene blue. Henceforth, several groups [12–16] investigated the photocatalytic and photo-electrochemical properties of nitrogen-doped TiO2 powders and thin films prepared by different methods.
Although the use of TiO2 in disinfection and the photocatalytic degradation organic pollutants has been studied extensively, there has been no report regarding the visible-light-induced bactericidal effects of TiO2 doped with a nonmetal element. The information about this visible-light induced disinfection is both scientifically and practically important. We believe that the nonmetal doping is a promising approach to improve the efficiency of TiO2 disinfection. In the present work, we reveal that nitrogen-doped TiO2 powder exhibits bactericidal effects on Escherichia coli under visible light irradiation.
2 Materials and methods
2.1 Preparation and characterization of N-TiO2
Nitrogen-doped TiO2 was prepared by sol–gel methods. Tetrabutyl titanate ((Ti(O(CH2)3–CH3)4, TBT) as Ti source and urea as nitrogen source were dissolved in absolute ethanol under vigorous stirring, respectively. And the pH value of solution was adjusted to 2–3 by addition of glacial acetic acid. After being stirred for 30 min, some water was dropping into the mixed solution with 1 mL/min, and the gel was obtained by hydrolysis. The gel was dried and thermal-treated to crystallize. The solid was ground to a powder with an agate mortar.
The X-ray diffraction (XRD, Bruker, D8 advance) patterns were used to identify the phase constitutions in samples and their crystallite size. UV–vis diffuse reflectance spectra were achieved by a UV–vis spectrophotometer (Shimadzu, UV3600). Morphologies of samples were characterized using a transmission electron microscopy (FEI, Tecnai 20). The XPS measurement was performed by an X-ray photoelectron spectrometer (VG, EscalabMK II).
2.2 Antimicrobial property evaluation of nanoparticles
We consulted ISO 22196-2007 and the National Standard GB/T 21510-2008 of the people’s republic of China to evaluate the antimicrobial property of TiO2 nanoparticles. E. coli ATCC 8099 was used as a model microorganism for all inactivation studies. It was assumed that one cell (or one cluster of cells) will multiply on the medium to produce a visible colony. One milliliter diluted suspension with a final cell concentration of 105 cfu/mL was mixed with the required amount of nano-TiO2 suspension, after a period then plating 0.5 mL of each mixed solution onto nutrient agar in triplicate, and counting the resultant colonies. The survival ratio of germ was calculated as follow formula:
R represents the survival rate of bacterium; N and N0 mean the colony number of each sample group and positive group, respectively.
The antibacterial property evaluation against E. coli was carried out under simulated sunlight and dark. The xenon lamp (300 W, 15 mA) was used as simulated sunlight with a cutoff filter (λ > 420 nm) to block the passage of UV light, and in dark the incubator was enveloped with aluminium foil.
Scanning electron microscopy (SEM, FEI, Quanta200) imaging was carried out as follows [17]: E. coli cultures in the late exponential growth phase (OD 0.7–0.9) exposed to nanoparticles were collected and fixed with 2.5 % glutaradehyde in phosphate buffer at pH 7.2 for 4 h. Fixed samples were rinsed in buffer. Dehydration of sample was carried out through increasing ethanol concentrations of alcohol and water solution from 30, 50, 70, 85, 95 % to absolute alcohol, then the obtained sample was fixed on microscope slide and coated with 1.2 nm of aurum in a sputter coater. Images were collected with an Apollo 300 field emission scanning electron microscope, operated at 10 kV.
3 Results and discussions
3.1 Characterization of N-TiO2
Figure 1 shows the UV–vis diffuse reflectance spectra of the undoped TiO2 and N-doped TiO2 samples. The undoped TiO2 is white in color and shows absorption only in the UV region (λ < 390 nm). The energy band gap estimated from the sharp absorption edge is 3.2 eV. The nitrogen-doped TiO2 samples are yellowy and their optical absorption was extended to the visible region. It is noted that the band gap was expanded from 380 to 480 nm upon N doping. Noticeable shifts of the absorbance shoulder from a wavelength below 400 nm to the visible light region were observed for the N-doped TiO2. The main absorption edges of the N-doped TiO2 change significantly compared to that of the undoped sample. It is likely that nitrogen doping creates a new N 2p state slightly above the valence band top consists of O 2p state, and this pushes up the valence band top and leads to visible light response as a consequence [18, 19]. Undoubtedly, these results reveal that the nitrogen element are indeed incorporated into the lattice of TiO2, thus altering its crystal and electronic structures.
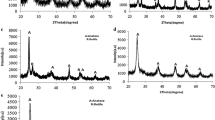
XRD patterns of the as-prepared nanophase undoped TiO2 and N-doped TiO2 powders are shown in Fig. 2. All diffraction peaks of undoped TiO2 and N-doped TiO2 can be assigned to anatase TiO2 and the diffraction data are in good agreement with the JCPDS card for titania (JCPDS No. No. 73-1764). The five major peaks locate at about 25.2°, 37.8°, 47.9°, 54.0°, and 62.7°. The crystal form of undoped TiO2 and N-doped TiO2 is anatase. Theoretically as the effective ionic radius of nitrogen (146 pm, consult the Lange’s Chemistry Handbook version 15th) is slightly over that of oxygen (138 pm, consult the Lange’s Chemistry Handbook version 15th), the doping could lead to the interplanar spacing becoming greater. However from the Fig. 2, comparing the diffraction angle of undoped TiO2 and N-doped TiO2, the differences are not distinguished.
As the penetrability of ultraviolet ray is less than X-ray, the UV–vis diffuse reflectance spectra reflect the optical absorption of particles surface layer, but the X-ray can penetrate through a certain range of thickness so that the XRD patterns can show the state of things inside the particles. Integrated the results of UV–vis scan and the XRD, the nitrogen doping is happened mostly on the surface layer of TiO2 particles, and majority of undoped TiO2 is in the particle core.
Transmission electron microscopy (TEM) was used to investigate the exact microstructure of samples. From Fig. 3, we can see that the nitrogen-doped TiO2 sample consists of uniform nanoparticles with average diameter of 20–30 nm.
To explore the states of the doped nitrogen species, the nitrogen-doped samples were subjected to X-ray photoelectron spectroscopy (XPS) analysis. Figure 4 shows the XPS survey spectrum of the undoped TiO2 and N-TiO2 sample. Obviously, Ti, O, and C elements exist at the surface of the sample, whereas the peak intensity is different. Figure 4 depicts the N 1 s XPS spectra of N-TiO2 with 20 scans, showing the peaks at the binding energy positions of 380–404 eV. After doping with N, two peaks are observed at binding energy of 398.5 and 401.2 eV. From previous reports [20–22], the binding energy peaks at 398.5 eV is attributed to anionic N doping incorporated into TiO2 as an O–Ti–N structural feature. The O–Ti–N linkage is a typical substitutional N doping, which has been reported to be due to N atoms within NH3 being bonded to Ti atoms and replacing lattice oxygen atoms in the TiO2. The higher binding energy peaks at 401.2 eV can be assigned to Ti–O–N. After the N-TiO2 specimen was etched by Ar ion for 300 s, a layer of about 10 nm thicknesses was removed from the particle surface, and the N 1 s peak for N doping exist still but it is less obvious and the intensity is weaken, so we think the nitrogen doping is happened mostly on the surface layer of particles, and majority of undoped TiO2 is in the particle core. From the high resolution XPS spectra for Ti 2p, it can be seen that Ti 2p spectra consists of two peaks at around 458.7 eV (Ti 2p3/2) and 464.2 eV (Ti 2p1/2). Ti 2p region showed that Ti was in +4 states, which was confirmed by the oxidation of Ti to TiO2. The peak at 458.7 eV becomes broader and unsymmetrical after N doping, in addition the binding energy is increased [23]. These indicate that a Ti +3 state is present due to the vacancies created by the nitrogen doping. However the change becomes indistinct after the N-TiO2 specimen was etched. So the N doping is happened on the surface layer of TiO2 particles, and producing numerous defects and lattice vacancies. But in the particle core, the undoped TiO2 is available in abundance. The nitrogen concentration can not be detected by only one scan at the surface of the sample for it is not homogeneous.
3.2 Antimicrobial property of N-TiO2 nanoparticles
The antibacterial activities of the samples were evaluated by the killing of E. coli in water under visible light irradiation on the basis of the decrease in the colony number of Escherichia coli formed on an agar plate.
According to the Figs. 5 and 6, the survival ratio of E. coli can be decreased to 8.75 % within 2 h N-doped TiO2 nanoparticles under visible light irradiation. Neither undoped TiO2 nor N-doped TiO2 in the dark for 24 h shows any bactericidal effects on E. coli, indicating that the photocatalyst itself is not toxic to E. coli. Thus, the bactericidal effect is ascribed to the photocatalytic reaction of the N-doped TiO2.
The photocatalytic antibacterial activity of the N-doped is obvious under visible irradiation. Visible light activity in N-doped TiO2 may be caused by band-gap narrowing from mixing the N 2p states with O 2p states. The phenomenon of band-gap narrowing in nonmetal atom doped TiO2 has been reported in the literatures [24, 25].
In addition, possible mechanisms for the bactericidal effect of TiO2 photocatalysis have been proposed. The photocatalysts are activated at the sub-band gap energy when exposed to visible light and generate reactive oxygen species such as hydroxyl radicals. These hydroxyl radicals cause various damages to living organisms [26]. Further study about the generation of hydroxyl radical levels from N-doped TiO2 under visible light irradiation is currently in progress.
From Fig. 7, we can see that the nanoparticles aggregated upon the cells, and the EDS characterization of the aggregate indicates that it was TiO2 (blue circle), some cells showed substantial damage (yellow circle points to the damaged part of the cell). It has been reported previously that the fullerene compounds might have caused the destruction of membrane integrity in bacteria [17]. Moreover, the aggregation of organism was associated with oxidative stress and aggregation could be a protection mechanism. On the other hand, bacteria may produce certain membrane proteins to strengthen its membrane structure in response to nanoparticle stress to reduce its membrane’s permeability. This observation was consistent with previous evidence that fullerene compounds intercalate into the cell wall and cell membrane in some Gram-negative bacteria.
4 Conclusions
In conclusion, the goals both of extending the TiO2 spectral response to the visible region and of improving its photocatalytic activity were realized by doped with nitrogen. The photocatalyst antibactericidal activity showed much higher photoreactivity than undoped TiO2 both on E. coli under visible light irradiation. This study demonstrated a novel approach for the efficient utilization of visible light in killing bacteria through doping nitrogen into TiO2 photocatalyst. The photo-catalytic antibacterial activity was activated under visible light, and the generated hydroxyl radicals from the N-doped TiO2 leaded to considerable bactericidal effects on E. coli. The structure and integrity of cell wall and cell membrane were destructed so that the bacteria were killed.
References
Matsunaga T, Tomoda R, Nakajima T, Wake H (1985) FEMS Microbiol Lett 29:211–214
Linsebigler AL, Lu G, Yates JT (1995) Chem Rev 95:735–758
In S, Orlov A, Berg R, García F, Jimenez SP, Tikhov MS, Wright DS, Lambert RM (2007) J Am Chem Soc 129:13790–13791
Irie H, Watanabe Y, Hashimoto K (2003) Chem Lett 32:772–773
Asahi R, Morikawa T, Ohwaki T, Aoki K, Taga Y (2001) Science 293:269–271
Czoska AM, Livraghi S, Paganini MC, Giamello E, Di Valentin C, Granozzi G (2011) Phys Chem Chem Phys 13:136–143
Umebayashi T, Yamaki T, Itoh H, Asai K (2002) Appl Phys Lett 81:454–456
Lin XX, Fu DG (2012) Adv Mater Res 374:895–898
Luo H, Takata T, Lee Y, Zhao J, Domen K, Yan Y (2004) Chem Mater 16:846–849
Wang Z, Cai W, Hong X, Zhao X, Xu F, Cai C (2005) Appl Catal B 57:223–231
Asahi R, Morikawa T (2007) Chem Phys 339:57–63
Shi H, Li X, Iwai H, Zhou Z, Ye J (2009) J Phys Chem Solids 70:931–935
Shao G, Wang F, Ren T (2009) Appl Catal B 92:61–67
Akpan UG, Hameed BH (2010) Appl Catal A 375:1–11
Shi H, Zhang T, Wanng H (2011) J Rare Earths 29:746–752
Tsao N, Luh TY, Chou CK (2002) J Antimicrob Chemother 49:641–649
Tang YJ, Ashcroft JM, Ding Chen (2007) Nano Lett 7:754–760
Nosaka Y, Matsushita M, Nishino J, Nosaka AY (2005) Sci Technol Adv Mater 6:143–148
Livraghi S, Paganini MC, Giamello E, Selloni A, Valentin CD, Pacchioni G (2006) J Am Chem Soc 128:15666–15671
Ou HH, Lo SL, Liao CH (2011) J Phys Chem C 115:4000–4007
Valentin CD, Finazzi E, Pacchioni G, Selloni A, Livraghi S, Paganini MC, Giamello E (2007) Chem Phys 339:44–56
Qiu X, Zhao Y, Burda C (2007) Adv Mater 19:3995–3999
Junna Xu, Liu Qing, Lin Shufeng, Cao Wenbin (2013) Res Chem Intermed 39:1655–1664
Lin L, Zheng RY, Xie JL, Zhu YX, Xie YC (2007) Appl Catal B 76:196–202
Akpan UG, Hameed BH (2010) Appl Catal A 375:1–11
Li X, Zhuang Z, Li W, Pan H (2012) Appl Catal A 429–430:31–38
Acknowledgments
This work was financially supported by the Fundamental Research Funds for the Central Universities (NO. NS2012113) and State Environmental Protection Key Laboratory of Microorganism Application and Risk Control open fund (No. MARC2011D043).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, P., Tao, J., Huang, X. et al. Preparation and photocatalytic antibacterial property of nitrogen doped TiO2 nanoparticles. J Sol-Gel Sci Technol 68, 213–218 (2013). https://doi.org/10.1007/s10971-013-3154-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-013-3154-y