Abstract
The effect of post-heat treatment process on TiO2 nanoparticles (NPs), prepared by hydrothermal-assisted sol-gel method, was investigated through measurement of the band gap energy, crystal size, photocatalytic activity and antibacterial efficiency of TiO2 NPs. X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR), Scanning electron microscopy (SEM) and UV-Vis spectroscopy were used to characterize treated as well as untreated TiO2 nanoparticles. Methylene blue (MB) dye was used as a pollutant model in order to investigate the photocatalytic activity of as-prepared TiO2 nanoparticles. The antibacterial activity of TiO2 nanoparticles was assessed under UV-A irradiation by testing the growth inhibition of two bacterial strains: a Gram negative bacteria Stenotrophomonas maltophilia (S. maltophilia) and a Gram positive bacteria Micrococcus luteus (M. luteus). The results indicate that the crystal size increases from 13 to 20 nm for untreated (NT) and treated nanoparticles, respectively. FTIR results show that heat treatment eliminates inorganic impurity. SEM micrographs prove that annealing does not modify the morphology of nanoparticles, however, particle size increases due to calcination. In addition, post-heat treatment at 500 °C for 2 h decreases the band gap energy (from 4.35 to 3.25 eV) and consequently enhances significantly the photocatalytic activity as well as the antibacterial performance of as-synthesized TiO2 nanoparticles.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Nanocrystalline titania (TiO2) powders are of high interest because of their attractive properties such as its non-toxicity, high chemical stability, low cost and strong oxidizing power [9]. Their unique features make them a competitive candidate for many applications such as photovoltaic cells, treatment of skin tumors or skin disease, killing of bacteria, anti-fogging and self-cleaning application [1, 3, 4]. Many studies confirmed that the anatase phase of titania showed the best photocatalytic activity which makes it widely used for air purification, dangerous waste treating and water treatment. [6, 10, 13]. However, the fast recombination of photogenerated electron-hole pair is still the main weakness of anatase TiO2 nanoparticles [7, 8]. Various studies have been developed in order to enhance the photo-response of TiO2 nanoparticles and reduce electron-hole recombination in the photocatalytic process. It is well known that preparing methods and post-treatment conditions strongly affect the photocatalytic activity of TiO2 because they have a critical influence on the physical and chemical properties of TiO2 [11]. Crystal structure, particle size in addition to surface area are considered as essential factors that affect the photoactivity of TiO2, on the other hand, heat treatment can be used to control physicochemical properties of TiO2 [2].
In this work, the effect of heat treatment on crystalline size, particle size, structure, band gap energy, photocatalytic and antibacterial activity of sol-gel prepared TiO2 nanoparticles have been investigated. Nanoparticles before and after heat treatment were characterized using X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR), Scanning electron microscopy (SEM) and UV-Vis spectroscopy. Photo-degradation and antibacterial tests have been also carried out in order to investigate the effect of calcination on the photocatalytic performances of TiO2 nanoparticles.
2 Materials and Methods
Sample Preparation. All the chemicals were of analytical grade and used without further purification. In order to prepare the TiO2 nanopowder, 5 ml of ethanol, 5 ml of acetic acid and 200 μL of hydrochloric acid used as catalysts in addition to 5 ml of titanium tetra isopropoxide precursor were mixed in the indicated order. The mixture was stirred for 15 min at a constant speed of 200 rpm to obtain the TiO2 sol. Then, the resulting sol was introduced into autoclave heated up to 243 °C and pressurized to overcome the critical point of ethanol (Tc = 243 °C, Pc = 63 bar). The sol gelation occurred after maintaining the temperature at 243 °C for 1 h. To evacuate the interstitial solvent, depressurization for 30 min down to room temperature is conducted with nitrogen gas. Finally, titanium aerogel was obtained. The obtained TiO2 nanopowders undergo heat-treatment at 500 °C for 2 h.
Characterization of as-prepared TiO2 nanoparticles. The structure and crystallite size of TiO2 nanoparticles were analyzed by means of the XRD measurements which were carried out at room temperature by using (BRUKER-AXS-D8-Advance) with CuKα radiation (λ = 0.154056 nm). The average crystallite size of TiO2 was calculated according to the Scherrer’s equation using the XRD line broadening as follows:
where D is the crystallite size (Å); k = 0.89; λ is the X-ray wavelength equal to 0.154056 nm; β is the full width at half maximum intensity (FWHM) and θ—half diffraction angle.
Infrared spectra were recorded at room temperature using a Thermo Nicolet 5700 spectrophotometer. Measurements were done via transmission mode. Scanning electron microscope (SEM) was performed in order to investigate the effect of heat treatment on the morphological and particle size of nanoparticles of TiO2. SEM images were collected by using MEB environmental QUANTA 250. Optical properties were investigated using UV-Vis spectroscopy (Optima SP-3000 plus) within the wavelength range of 200–700 nm.
Dye Photodegradation. Photocatalytic tests were carried out to evaluate the photodegradation of methylene blue (MB) dye in the presence of TiO2 samples in aqueous solution under UV light. UV-A irradiation was performed by using three UV lamps with a maximum intensity at 365 nm. The intensity of UV radiation was found to be 0.76 mW/cm2. The distance between the radiation source and the solution was 10 cm.
Antibacterial test. Antibacterial activity of untreated and treated TiO2 nanoparticles suspensions (0.1 and 1% w/v) was performed under UV-A light, the test was carried out against two kinds of strains one Gram-negative bacteria (S. maltophilia BC656) and one Gram-positive bacteria (Micrococcus luteus BC657). The source of UV-irradiation was a 25 W fluorescent lamp with a maximum intensity at 365 nm. All strains were isolated and kept in the bacterial collection of the Department of Biological and Environmental Sciences (DISBA) of University of Messina, Italy. Fresh bacterial suspensions were prepared after growth in TSA medium (Tryptone Soy Agar, Oxoid) for 24–48 h and then adjusted at a final concentration of 1.5 × 107 cell/mL for Gram negative strain and 1.5 × 105 cell/mL for Gram positive strain. For the experiment, two different kinds of controls were used:
-
1.
Bacterial suspension alone exposed under UV light and considered as a control to test the effect of UV irradiation on tested bacteria noted ‘Cuv’;
-
2.
Another set of experiment with only bacteria not exposed to the UV was used as a control noted ‘C’ in order to check the viability of cells during the experimental time scale.
After 1 h of UV exposure and in order to evaluate the percentage of surviving cells, 10 µL of suspension and respective decimal dilutions were inoculated on the surface of Petri dishes containing TSA medium and then the percentage of surviving cells was estimated by counting the colonies after incubation at 28 °C for 24–48 h.
3 Results and Discussion
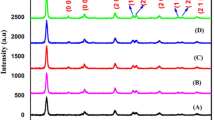
To determine the crystalline structure of as-prepared nanoparticles, XRD analysis was performed on both untreated and treated TiO2 (Fig. 1). All samples were pure anatase, and no rutile phase was detected. However better crystallinity after annealing TiO2 nanoparticles at 500 °C was observed. On the other hand, the average size of TiO2 nanopowder increased from 13 nm to be about 20 nm for untreated and annealed sample, respectively. From these results, it could be concluded that post heat treatment enhances the crystallinity of TiO2 nanoparticles and slightly increases their crystal size.
The FTIR spectrum of the as-synthesized TiO2 nanoparticles is given in Fig. 2. The band around 437 cm−1 is attributed to the O-Ti-O band corresponding to the crystalline titania in the anatase form. In the case of untreated sample, picks observed at the wavenumber range between 1300 and 1800 cm−1, were attributed to C = O, υ C = C and CH3 vibration corresponding to the organic residues. A very low band, in the higher wavenumber range, between 2700 and 3800 cm−1 was attributed to O-H corresponding to the surface adsorbed water and C-H vibrations. However, the as mentioned peaks are not observed in the case of treated which proves that heat treatment removes organic impurities and adsorbed water which in turn could enhance the MB adsorption on the surface of TiO2 nanoparticles and consequently ameliorate the photodegradation performances.
Scanning electron microscope (SEM) was performed in order to determine the effect of calcination on the morphology and particles size of TiO2 nanoparticles. Figure 3 shows the SEM micrographs of TiO2 particles and present spherical shape particles. It could be seen through the SEM analysis that TiO2 spheres of 1 μm consist of a large amount of mono-dispersed crystallites with a size between 20 and 40 nm. Moreover, it is observed that heat treatment does not affect the morphology of nanoparticles which remain spherical after annealing. However, it is clear that particle size increased after calcination which is consistent with the XRD result.
Figure 4 shows the UV-Vis absorbance spectra of samples. The band gap energy (Eg) of TiO2 nanoparticles before and after heat treatment process was determined by using the following equation:
where h is Planck’s constant, B is a constant dependent on the transition probability, and ν is the frequency of the radiation and α is the optical absorption coefficient.
It can be clearly seen from Fig. 4 that the absorbance of UV light was significantly enhanced due to heat treatment. On the other hand, results indicate that annealing TiO2 at 500 °C affect the optical band gap energy which decreased from 4.35 to 3.25 eV (inset of Fig. 4).
Photodegradation of MB dye was performed to investigate and compare the photocatalytic performance of treated and untreated TiO2 nanoparticles. Figure 5 shows that not treated TiO2 nanoparticles do not exhibit any significant photocatalytic activity by degrading only 10% of dye after 6 h. However, photocatalytic degradation rate was notably enhanced after treated nanoparticles at 500 °C. It is clear that calcination has a great effect on the photocatalytic activity of TiO2 nanopowders. The high photocatalytic performance of annealed TiO2 nanoparticles could be attributed to a several factors: (i) better UV-light absorption of the catalyst because of higher crystallinity and reduced band gap (ii) better adsorption of MB after the removal of organic impurities resulting from used precursors and which remain after sol-gel process (iii) the increase in the number of active sites and in the amount of photons absorbed by TiO2.
From the results shown in Fig. 6, we can conclude that UV light has no effect on inhibiting the growth of tested bacteria. Indeed, no reduction in the percentage of survival cell was observed after 1 h of exposure under UV-A radiation. This may be due to the low intensity of the used UV lamp. Consequently, we can determine that TiO2 nanoparticles are the only responsible for antibacterial activity and inactivation of bacterial growth for both bacteria. Calcined samples showed more efficient photocatalytic inactivation than not treated samples due to their enhanced photocatalytic activity. On the other hand, it is clear that 0.1% w/v suspension contains treated powders showed better antibacterial activity than not treated samples (1% w/v). This result reveals the importance of the calcination on photocatalytic activity of TiO2. In fact, it has been reported that the photocatalytic activity of TiO2 is greatly affected by the calcination temperature [5, 12]. On the other hand, 1% w/v calcined suspension showed the highest antibacterial action against gram negative as well as gram positive bacteria with approximately a total inhibition after 1 hour of contact with TiO2 nanoparticles under UV light; which exhibit their effectiveness in determining practically the complete killing of bacteria tested.
4 Conclusion
The results of this work point out that the heat treatment strongly affects the photocatalytic performance of TiO2 nanoparticles, in particular, the photocatalytic efficiency and antibacterial activity against. Enhancing the absorbance under UV light and narrowing the band gap energy of heat-treated TiO2 nanoparticles are the main reasons for efficient photo-response activity. The removal of organic impurities from TiO2 NPs with heat treatment was beneficial to the studied application. The crystal size as well as particle size increased due to calcination. However, the calcination has no effect on particles morphology which remains spherical. Treated TiO2 nanoparticles seem to be a promising candidate for many applications such as self cleaning surfaces, air purification and water treatment.
References
Bai Y, Mora-Seró I, De Angelis F et al (2014) Titanium dioxide nanomaterials for photovoltaic applications. Chem Rev 114:10095–10130. https://doi.org/10.1021/cr400606n
Behnajady MA, Alamdari ME, Modirshahla N (2013) Investigation of the effect of heat treatment process on characteristics and photocatalytic activity of TiO2-UV100 nanoparticles. Environ Prot Eng 39:33–46. https://doi.org/10.5277/EPE130103
Cai R, Van GM, Aw PK, Itoh K (2006) Solar-driven self-cleaning coating for a painted surface. C R Chim 9:829–835. https://doi.org/10.1016/j.crci.2005.04.007
Fujishima A, Rao TN, Tryk DA (2000) Titanium dioxide photocatalysis. J Photochem Photobiol C Photochem Rev 1:1–21. https://doi.org/10.1016/S1389-5567(00)00002-2
Guillard C, Beaugiraud B, Dutriez C et al (2002) Physicochemical properties and photocatalytic activities of TiO2-films prepared by sol–gel methods. Appl Catal B Environ 39:331–342. https://doi.org/10.1016/S0926-3373(02)00120-0
Kawahara T, Ozawa T, Iwasaki M et al (2003) Photocatalytic activity of rutile-anatase coupled TiO2 particles prepared by a dissolution-reprecipitation method. J Colloid Interface Sci 267:377–381. https://doi.org/10.1016/S0021-9797(03)00755-0
Mahalakshmi M, Arabindoo B, Palanichamy M, Murugesan V (2007) Preparation, Characterization, and Photocatalytic Activity of Gd3+ Doped TiO2 Nanoparticles. J Nanosci Nanotechnol 7:3277–3285. https://doi.org/10.1166/jnn.2007.689
Rockafellow EM, Stewart LK, Jenks WS (2009) Is sulfur-doped TiO2 an effective visible light photocatalyst for remediation? Appl Catal B Environ 91:554–562. https://doi.org/10.1016/j.apcatb.2009.06.027
Saif M, El-Molla SA, Aboul-Fotouh SMK et al (2014) Nanostructured Gd3+-TiO2 surfaces for self-cleaning application. J Mol Struct 1067:120–126. https://doi.org/10.1016/j.molstruc.2014.03.024
Vamathevan V, Amal R, Beydoun D et al (2002) Photocatalytic oxidation of organics in water using pure and silver-modified titanium dioxide particles. J Photochem Photobiol A Chem 148:233–245. https://doi.org/10.1016/S1010-6030(02)00049-7
Yu J, Yu H, Cheng B et al (2006) Enhanced photocatalytic activity of TiO2 powder (P25) by hydrothermal treatment. J Mol Catal A Chem 253:112–118. https://doi.org/10.1016/j.molcata.2006.03.021
Yu J, Yu JC, Ho W, Jiang Z (2002) Effects of calcination temperature on the photocatalytic activity and photo-induced super-hydrophilicity of mesoporous TiO2 thin films. New J Chem 26:607–613. https://doi.org/10.1039/b200964a
Yu JC, Yu J, Zhang L, Ho W (2002) Enhancing effects of water content and ultrasonic irradiation on the photocatalytic activity of nano-sized TiO2 powders. J Photochem Photobiol A 148:263–271
Acknowledgements
The authors gratefully acknowledge the helpful comments and suggestions of the reviewers, which have improved the presentation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this paper
Cite this paper
Ben Chobba, M., Messaoud, M., Bouaziz, J., De Leo, F., Urzì, C. (2020). The Effect of Heat Treatment on Photocatalytic Performance and Antibacterial Activity of TiO2 Nanoparticles Prepared by Sol-Gel Method. In: Chaari, F., et al. Advances in Materials, Mechanics and Manufacturing. Lecture Notes in Mechanical Engineering. Springer, Cham. https://doi.org/10.1007/978-3-030-24247-3_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-24247-3_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-24246-6
Online ISBN: 978-3-030-24247-3
eBook Packages: EngineeringEngineering (R0)