Abstract
Ferrites of composition M0.2Co0.4Zn0.4Fe2O4 with M = Cu2+, Mn2+ and Ni2+ were prepared by citrate complex method. Later, their composites with silica have also been obtained by a simple route. The citrate complex precursors of multielement ferrites were characterized by FTIR spectroscopy and thermal analysis, been found a similar behavior for the three systems. The thermal treatment (at 400, 600 and 800 °C) of precursors gives, as result, the spinel type cubic ferrite pure when the ions substituted were copper and nickel; when manganese was used an hematite phase was obtained as contaminant at 800 °C. The presence of all ions involved and the particle size was corroborated by EDX analysis and measured from a TEM micrograph, respectively. The magnetic parameters related to magnetic properties, magnetization and coercivity, were different depending of the chemical composition of the ferrite and the thermal treatment temperature, as it was expected. At room temperature, the values obtained were near to those reported for Co-ferrite in bulk. The synthesis route of the composites M0.2Co0.4Zn0.4Fe2O4-SiO2, proposed in this work, gives as result magnetic nanoparticles in an amorphous silica matrix. Their magnetic properties were depending on weight percentage of the magnetic phase in the composite.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Traditionally soft ferrites (MFe2O4) have been used in a broad variety of applications such as magnetic storage devices, core materials for power transformers, antenna rods, loading coils, etcetera [1]. The use of chemical routes for their synthesis have allowed to obtain ferrites in the nanometric scale, which present magnetic, electric, catalytic and mechanical properties outstandingly improved with respect to the existing materials [2, 3]. Nanometric soft ferrites are nowadays used, moreover, as microwave absorbents, magnetically guided drug deliverers, ferrofluids, multilayer chip inductors, electromagnetic interference filters, etc. [4]. An additional advantage of some chemical routes is the possibility to control the stoichiometry of the products [5]. In this sense, multielement ferrites, principally those based on Ni–Zn or Mn–Zn have been synthesized and have shown prominent properties [6, 7]. Actually, exist a growing interest in synthesize ferrites substituted by more than two ions because they have shown, in some cases, coupling phenomena and interactions between electrical current and magnetism [8], which is interesting from a theoretical point of view, and give place to many potential future applications, e.g. magnetologic devices [9], photonic systems [10], spintronic devices [11], biomedical systems [6, 12] by mention some. An important fact is the trend of small particles to agglomerate, in order to reduce the energy associated with their high surface area. To minimize this, dispersing or embedding the nanoparticles into a silica matrix has been a successful method [12]. Some advantages of synthesizing multielement ferrites into a silica matrix are: chemical stability, strong magnetization, low coercivity, biological or electronic compatibility provided by the good possibility of functionalize the silica, etc.
For all these reasons, we have carried out the synthesis of three different ferrites of M0.2Co0.4Zn0.4Fe2O4 composition by the citrate complex method, and we have systematically studied the effect, on the magnetic properties, of the M substitution by different divalent ions (Cu2+, Mn2+ or Ni2+). Moreover, this paper reports a simple mechanochemical approach that provides a resolution of several important problems in the synthesis of magnetic nanoparticles-silica composites by Stöber process related to the necessity of silicate pretreatment and its slow kinetics [13].
2 Materials and methods
The chemical reagents used in this work were citric acid monohydrate, ethylene glycol, Cu(NO3)2·6H2O, Mn(NO3)2·6H2O, Ni(NO3)2·6H2O, Co(NO3)2·6H2O, Zn(NO3)2·6H2O, Fe(NO3)3·9H2O, tetraethylorthosilicate (TEOS), oleic acid, heptane, purchased from Aldrich. All chemicals were analytical grade (purity >99.9%) and used without further purification.
2.1 Synthesis of M0.2Co0.4Zn0.4Fe2O4
In general the citric acid was mixed with ethylene glycol, at room temperature under magnetic stirring until a transparent solution was obtained, in a molar relation 1–4, respectively. Then appropriate amount of iron nitrate (2 mol), zinc nitrate (0.4 mol), cobalt nitrate (0.4 mol) and, finally, copper, manganese or nickel nitrate (0.2 mol) were added into the solution, one by one, in that order, ensuring the complete dissolution before add the next salt. Homogeneous citrate complex solutions were obtained. Every solution was heated at ~130 °C by 2 h to increase the viscosity as a consequence of the evaporation of solvent in excess and water produced by condensation reaction between citric acid and ethylene glycol. The precursors were casted on Petri dishes and treated at 130 °C by 72 h in an oven. Dark brown vitreous films were obtained and, then, grounded into powder and calcined at 400, 600 and 800 °C by 2 h under air atmosphere. A flowchart of this procedure is shown in Scheme 1.
2.2 Synthesis of M0.2Co0.4Zn0.4Fe2O4-SiO2 composites
The composites based on magnetic nanoparticles, M0.2Co0.4Zn0.4Fe2O4 with M = Cu, Mn or Ni, and an amorphous silica matrix were produced by high energy ball milling. Into the vial were collocated: balls (~50 g), as milling medium; M0.2Co0.4Zn0.4Fe2O4 (5 g), as magnetic nanoparticles; oleic acid (15 mL), as surfactant; and heptane (30 mL), as solvent. The vial and balls (15 mm) were made of agate. The blends were subjected to a rotational speed of 200 rpm by 2 h. During 1 h the sample was left in repose. This procedure was repeated 3 times. After this, 10 mL of TEOS were added to the blend and milled again at 200 rpm by 2 h with 1 h in repose by three times. Finally, the heptane excess was removed at 50 °C by 2 h in an oven.
2.3 Characterization techniques
The identification of citrate complexes and ester groups in the precursors were studied by FTIR spectroscopy, by transmission through KBr pellets containing about 1% of sample, using a Thermo-Nicolet/Nexus 470 spectrometer.
The thermal behavior of precursors was followed by thermogravimetric analysis, in a Shimadzu TGA-50, from 25 to 800 °C with a heating rate of 10 °C/min.
The crystalline phases were identified by XRD on a SIEMENS D-5000 powder diffractometer (25 mW, 35 kV) in the 10–80 2θ (°) range with a step of 0.02 °/s, using CuKα radiation (λ = 0.15418 nm). The crystallite mean size was deduced from XRD line integral breath β using Scherrer’s equation [14]. The particle size, crystallinity and composition of ferrite nanoparticles and composite (ferrites-SiO2) were characterized by high resolution transmission electron microscopy (HRTEM) coupled with energy-dispersive X-ray spectroscopy. A field-emission electron-source TEM (FEI-TITAN) was operated at 300 kV. The samples were deposited on a gold-grid-supported perforated transparent carbon foil.
The magnetic properties of the nanoparticles and their composites were measured, at 3 K and room-temperature, on a Quantum Design SQUID with VSM option. The magnetization values are given per gram of sample.
3 Results and discussion
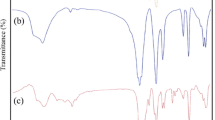
FTIR spectra of precursors synthesized to obtain the multielement ferrites, M0.2Co0.4Zn0.4Fe2O4, present characteristic absorption bands similar even when the solutions were heated at 130 °C for 24, 48 or 72 h. Figure 1 shows the FTIR spectrum of the Cu0.2Co0.4Zn0.4Fe2O4 precursor (130 °C/24 h) as an example. The broad band at ~3,400 cm−1 is related to the stretching vibration (ν) of O–H bonds present in the polyester obtained from the reaction between CA and EG, in metallic salts presence, and the EG in excess trapped into the polymeric chains. Thin bands at 1,650, 1,380 and 1,247 cm−1 are due to stretching modes (ν) of C = O and C–O when the carboxylate groups are bonded to metallic ions. Small signals at 895, 790 and 530 cm−1 are attributable to bending mode (δ) of the same bonds in the carboxylate groups. All this is supported by analogy with evidence from FTIR and NMR spectroscopy reported previously for similar systems [15, 16].
Figure 2 shows a characteristic thermogram (TGA) of the M0.2Co0.4Zn0.4Fe2O4 precursors, in specific that obtained from Mn0.2Co0.4Zn0.4Fe2O4 precursor (130 °C/72 h). As can be observed the precursors degrade through two critical steps. The first step of weight loss corresponds to the gradual evaporation of adsorbed water and EG trapped into polymeric network in the range of 25–300 °C. The second weight loss is due to a fast degradation of polyester (formed through a Fisher esterification reaction between CA and EG, the polymeric product is due to the functionality of the reagents used) which finish at ~320 °C. A total weight loss of ~60% was observed, for all samples, and attributed to organics present in the precursors. DTA curves (not included in this paper) show that first step is an endothermic reaction whereas the decomposition is an exothermic reaction. The absence of a second exothermic peak indicates that decomposition and crystallization occur simultaneously over the temperature range of 300–320 °C. So, thermal treatments from 400 °C are necessary and helpful to complete the ferrite crystallization process from the precursors synthesized.
Figure 3 shows, as an example, the XRD patterns of Ni0.2Co0.4Zn0.4Fe2O4, obtained at 400, 600 and 800 °C. All the broad characteristic peaks associated to a spinel-type cubic structure [17], PDF 22-1086, appear except the XRD diagram corresponding to sample Ni0.2Co0.4Zn0.4Fe2O4, at 400 °C, which shows only a broad maximum of low intensity.
In general, it is appreciable how the diffraction peaks become sharper as the treatment temperature increase, revealing the larger size of nanocrystals. A high temperature for Mn2+, in that sample treated at 800 °C, produce an inhomogeneous sample (with its decomposition [18]) and the crystallization of Fe3+ in excess, as hematite (~30%W of Fe2O3 PDF 33-0664), in addition to spinel-type cubic structure. As can be observed in Table 1, where they are tabulated the composition, structural parameters and average crystallite sizes. The lattice parameter a obtained for Cu0.2Co0.4Zn0.4Fe2O4 and Ni0.2Co0.4Zn0.4Fe2O4 (8.3964 Å) is between those reported values of 8.3919 and 8.4409 Å for CoFe2O4 and ZnFe2O4, respectively (PDF 22-1086 and 82-1049). By contrast, Mn0.2Co0.4Zn0.4Fe2O4 shows a different lattice parameter value depending of the temperature. This could be attributed, again, to a lack of homogeneity in this sample as a consequence of a high temperature [18]. The crystallite size range calculated, using a Gaussian and Lorentzian peak shape of equal contribution on the most intense reflection (h k l = 3 1 1), vary from 13.6 to 63.8 nm. The increase of this value is a consequence, mainly, of the increase in the temperature of thermal treatment of the precursors. It is important to mention that the presence of all cations in the structure was corroborated by EDX analysis.
The room-temperature magnetization curves of the studied multielement ferrites, treated at 600 and 800 °C, display the typical ferrimagnetic behavior; with large saturation magnetization, in the range found for bulk Co-ferrite [19], although had less coercivity. As an example, Fig. 4 shows the magnetization curves of ferrites obtained at 600 °C. The ferrimagnetic nature is corroborated by the magnetization measurements under zero-field cooling conditions where a continuously increasing until 300 K is observed for Cu0.2Co0.4Zn0.4Fe2O4, Mn0.2Co0.4Zn0.4Fe2O4 or Ni0.2Co0.4Zn0.4Fe2O4 [17]. In agreement with the difference observed in particle size, at 600 °C for the Mn0.2Co0.4Zn0.4Fe2O4, shows a smaller saturation magnetization value than that reached for Cu0.2Co0.4Zn0.4Fe2O4 and Ni0.2Co0.4Zn0.4Fe2O4.
As its well known, low temperature magnetization measurements provide, at temperatures close to 0 K, information about distribution of cations within the spinel lattice. Namely, at 0 K, saturation magnetization directly reflects the difference in magnetization of the tetrahedral and the octahedral sublattices [17]. A lowest degree of inversion leads to largest values of saturation magnetization due to that magnetic moment per molecule of CoFe2O4 increases from 3 Bohr magnetons for inverse spinel to 7 Bohr magnetons for normal spinel [20]. Figure 5 shows hysteresis loops, measured at 3 K, of M0.2Co0.4Zn0.4Fe2O4 composition nanoparticles obtained at 600 °C, of the three studied systems, Cu0.2Co0.4Zn0.4Fe2O4 shows the largest saturation magnetization at 3 K (125.5 emu/g), which suggests a normal spinel type structure. Other reason for inferring a normal spinel is that the presence of M2+ in tetrahedral sublattice sites strongly reduces the coercivity from that would be expected if the M2+ was occupying octahedral sites [21]. The Mn0.2Co0.4Zn0.4Fe2O4 nanoparticles (600 °C) present a similar behavior reaching a Ms value of 113.7 emu/g at 3 K. However, there is not difference in saturation magnetization values of Ni0.2Co0.4Zn0.4Fe2O4, between that obtained at room temperature (74.9 emu/g) and 3 K (74.8 emu/g), which suggests an inverse spinel type structure. The distribution of cations in the structure depends of many factors as synthetic method, heat treatment temperature, heat-duration time, cooling rate and, how occurs on this case, the nature of the substituents [22]. Indeed, to determine the percentage of each element over the tetrahedral or octahedral sites will be necessary to analyze the samples by Mossbauer or K-edge x-ray absorption spectroscopy.
Figure 6 shows the XRD patterns of the materials after the mechanical milling of M0.2Co0.4Zn0.4Fe2O4 nanoparticles with TEOS, under the conditions reported in the experimental part. The spinel phase expected is obtained in all the cases, as well as, a broad maximum of low intensity, at ~20° (2θ), associated to the presence of SiO2 in amorphous state.
TEM images corresponding to the Cu0.2Co0.4Zn0.4Fe2O4 nanoparticles (left) and Cu0.2Co0.4Zn0.4Fe2O4-SiO2 composite (right) are shown in Fig. 7. After heating nanoparticles at 800 °C/2 h the images show agglomerated particles, which are still nanometer-sized, with the typical polygonal shape and an average diameter which increases up to around 70 nm in agreement with a progressive sintering process. These results imply that the heat energy supplied to the system not only induces the crystallization (observed by XRD) but also increases the surface energy of the particles favoring their aggregation. The procedure followed to obtain the composites does not induce the collapse of the magnetic phase as can be noticed at the right side image. Also, is evident, the presence of a silica matrix recovering the agglomerates.
The composites M0.2Co0.4Zn0.4Fe2O4-SiO2 show the ferrimagnetic behavior expected although the saturation magnetization values are decreased, with respect to that of M0.2Co0.4Zn0.4Fe2O4, as a consequence of the non magnetic contribution of SiO2. Two representative hysteresis loops, at room temperature and 3 K, are shown in Fig. 8 and the Ms and Hc values found for all systems are summarized in Table 2.
4 Conclusions
The chemical route followed in this work lead to obtain the multielement ferrites, Cu0.2Co0.4Zn0.4Fe2O4, Mn0.2Co0.4Zn0.4Fe2O4 and Ni0.2Co0.4Zn0.4Fe2O4, with nanometer particle size and magnetic properties in the range found for CoFe2O4 in bulk.
A simple mechanical milling route is useful in the obtaining of composites M0.2Co0.4Zn0.4Fe2O4-SiO2.
Abbreviations
- CA:
-
Citric acid
- EG:
-
Ethylene glycol
References
Jiles D (1998) Introduction to magnetism and magnetic materials. Chapman and Hall, Iowa
Jalaly M, Enayati MH, Kameli P, Karimzadeh F (2010) Phys B 405:507–512
Tan X, Li G, Zhao Y, Hu C (2010) J Alloy Compd 493:55–63
Cui H, Jia Y, Ren W, Wang W (2010) J Sol Gel Sci Technol 55:36–40
Kakihana M (1996) J Sol Gel Sci Technol 6:7–55
Nam JH, Joo YH, Lee JH, Chang JH, Cho JH, Chun MP, Kim BI (2009) J Magn Magn Mater 321:1389–1392
Ju Y-W, Park J-H, Jung H-R, Cho S-J, Lee W-J (2008) Compos Sci Technol 68:1704–1709
Petracic O (2010) Superlattices Microstruct 47:569–578
Allwood DA (2005) Science 309:1688–1692
Lyubchanskii IL, Dadoenkova NN, Lyubchanskii MI, Shapovalov EA, Rasing Th (2003) J Phys D Appl Phys 36:R277–R287
Battle X, Labarta A (2002) J Phys D 35:R15–R42
Morel AL, Nikitenko SI, Gionnet K, Wattiaux A, Lai-Kee-Him J, Labrugere C, Chevalier B, Deleris G, Petibois C, Brisson A, Simonoff M (2008) ACS Nano 2:847–856
Conrad CF, Icopini GA, Yasuhara H, Bandstra JZ, Brantley SL, Heaney PJ (2007) Geochim Cosmochim Acta 71:531–542
Cullity BD (1978) Elements of X-ray diffraction. Addison-Wesley, USA
Montemayor SM, García Cerda LA, Torres-Lubián JR, Rodríguez-Fernández OS (2008) Mater Res Bull 43:1112–1118
Montemayor SM, García Cerda LA, Torres-Lubián JR, Rodríguez-Fernández OS (2008) J Sol Gel Sci Technol 42:181–186
Gyergyek S, Makovec D, Kodre A, Arcon I, Jagodic M, Drofenik M (2010) J Nanopart Res 12:1263–1273
Sharma P, Gupta A, Owens FJ, Inoue A, Rao KV (2004) J Magn Magn Mater 282:115–121
Smit J, Wijn HPJ (1959) Ferrites. Philips’ Technical Library, Eindhoven
Kodama RH (1999) J Magn Magn Mater 200:359–372
Franco A, Zapf V (2008) J Magn Magn Mater 320:709–713
Gajbhiye NS, Balaji G, Bhattacharyya S, Ghafari M (2004) Hyperfine Interact 156/157:57–61
Acknowledgments
Financial support from Secretaría de Educación Pública through of the project UACOAH-PTC-132 is gratefully acknowledged. A. A. Rodríguez-Rodríguez thanks the scholarship IOO-13-SNI-Estudiantes-2008-01.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodríguez-Rodríguez, A.A., Rodríguez-Fernández, O.S., Osuna-Alarcón, J.G. et al. Synthesis of multielement ferrites and their silica composites. J Sol-Gel Sci Technol 61, 534–540 (2012). https://doi.org/10.1007/s10971-011-2656-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-011-2656-8