Abstract
Spinel CoFe2O4 nanoparticles were synthesized by a simple, economical, and eco-friendly hydrothermal method (HM) using metal nitrates and polyvinyl pyrrolidone. The structural, morphological, and magnetic properties of the products were determined and characterized in detail by X-ray diffraction (XRD), high-resolution scanning electron microscopy (HR-SEM), energy-dispersive X-ray spectroscopy (EDX), photoluminescence (PL) spectroscopy, vibrating sample magnetometry (VSM), and Brunauer–Emmett–Teller (BET) surface area analysis. Key parameters influencing the structural performance, such as particle size and shape, annealing temperature, functionalization, and magnetic properties, have been comprehensively discussed. The effect of the catalyst and solvent on the catalytic oxidation of benzyl alcohol using the CoFe2O4 nanoparticle catalyst prepared by hydrothermal method was also investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Interest in nanosized spinel ferrites has greatly increased in the past few years due to their importance in the understanding of the fundamentals of nanomagnetism. Transition metal spinel oxides (TMSO) are technologically important as magnetic materials, semiconductors, pigments, and effective catalysts for a number of industrial processes [1–4].
The maximum common type of ferrites was cubic spinel with the formula AB2O4, having tetrahedral (A site) and octahedral (B site) crystalline sites. Superparamagnetism is one of the key characteristics essential for the use of such materials in the medical field. Furthermore, the properties of spinel ferrite also depend on the crystallite size, morphology, and inherent cation distribution between the tetrahedral (A) and octahedral (B) sites or alteration of cation distribution due to the small amount of dopant [5–9].
Currently, by decreasing the size of the particles, the saturation magnetization regularly decreases due to noncollinear spin arrangements on or near the surface of the particles, which in turn affects the response of the substantial external magnetic field. Meanwhile, important properties of the spinel ferrites depend crucially on the exact nature of the cation distribution over the A and B sites; different substitutions have been incorporated to get the desired electrical and magnetic properties [10–14].
Several synthesis approaches including solvothermal, microemulsion, thermal decomposition, sol–gel, combustion, co-precipitation, ball milling, hydrothermal, and laser deposition methods are reported for the synthesis of spinel CoFe2O4 [15–18]. Compared with other methods, the hydrothermal synthesis seems to be the most promising because of its simplicity, productivity, and control of several factors such as size, morphology, composition, and agglomeration degree by changing the experimental conditions (temperature, time, reactants, and stirring rate) [19].
The novelty of the present work is that the method offers several advantages of high flexibility, reproducibility of quality, low-cost production, and ease of handling. It has a significant capability to control the particle size with high purity resulting in desirable properties of nanoparticles. In conclusion, the samples were tested for their catalytic activity towards the oxidation of benzyl alcohol.
2 Methodology
2.1 Materials
The metal salts of iron nitrate Fe(NO3)3⋅ 9H2O (99 %) and cobalt nitrate Co(NO3)2⋅ 6H2O (99 %) were used as metal precursors, polyvinyl pyrrolidone (PVP, MW = 29,000) as a capping agent for controlling the agglomeration of particles, and deionized water as a solvent. All chemical reagents were purchased from Sigma-Aldrich and used without further purification.
Cobalt nitrate and ferric nitrate were dissolved in deionized water and then mixed with polyvinyl pyrrolidone under constant stirring for 5 h, at room temperature, until a clear transparent solution was obtained. The final solution was transferred to a 500-ml Teflon-lined stainless steel autoclave. The sealed autoclave was allowed to maintain at 200 ∘C for 8 h, then cooled down to ambient temperature. The final products were washed repeatedly with distilled water and absolute ethanol several times and then dried at 60 ∘C. Later, these powders were sintered at 400, 500, and 600 ∘C at a heating rate of 5 ∘C/min for 5 h in an air atmosphere and the samples were labeled as CF1, CF2, and CF3.
2.2 Instruments and Characterization of Cobalt Ferrites
The structural studies were carried out using a Philips X’ pert diffractometer for 2𝜃 values ranging from 10∘ to 80∘ using Cu Kα radiation at λ = 0.154 nm. Morphological studies and energy-dispersive X-ray analysis of nanomaterials have been performed using a Jeol JSM 6360 high-resolution scanning electron microscope and Stereo-scan LEO 440 and a high-resolution transmission electron microscope (HR-TEM). The samples were coated with gold by a gold-sputtering device for better visibility of the surface morphology. The emission properties were recorded using a Varian Cary Eclipse Fluorescence spectrophotometer. Vibrating sample magnetometry (VSM) measurements were performed by using a Model 9600 vibrating sample magnetometer from LDJ Electronics Inc. The magnetization measurements were carried out in an external field up to 10 kOe at room temperature. The nitrogen adsorption–desorption isotherms of the samples were measured by using an automatic adsorption instrument (Quantachrome Quadrawin gas sorption analyzer) for the determination of the surface area and total pore volume.
3 Results and Discussion
3.1 X-ray Diffraction Studies
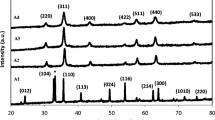
Figure 1 shows the X-ray diffraction patterns which were evidences of the formation of mono phases of the cobalt ferrites at calcination temperatures 400, 500, and 600 ∘C (CF1, CF2, and CF3). The broadening of the diffraction peak reveals the formation of the nanocrystalline phase. The peaks at 2𝜃 values of 18.59∘, 31.14∘, 35.74∘, 42.02∘, 57.53∘, and 62.81∘ are indexed to (111), (220), (311), (400), (511), and (440) reflection planes of cobalt ferrite, respectively. These values match well the (JCPDS card number 22-1086) signifying the cubic spinel structure with the Fd3m space group [20]. The results also showed that the diffraction peaks are sharper and narrower with their intensity increased with an increase of the calcination temperature. This indicates intensification in crystallinity due to the size enlargement of the nanoparticles.
The average crystallite size was obtained from X-ray diffraction (XRD) peaks using the Scherrer equation [21]
where L = average crystallite size (Å), λ = wavelength of the incident X-ray beam (1.54 Å), β= the FWHM (rad), and 𝜃 = is Bragg’s angle. The average crystallite size of samples A and B was found to be 20.9 (CF1), 25.6 (CF2), and 29.2 nm (CF3), respectively. The broad XRD lines indicate that the particles are of nanosize range. This figure demonstrates that the lattice parameter increases from 8.374 to 8.387 A ∘. Spinel-type magnetite shows a lattice parameter with reduction in crystallite size [22–25]. Size-dependent unit cell parameters of CoFe2O4 samples have been also reported by some research groups [26–28].
3.2 SEM Images
The mean particle size and their distribution were determined by analyzing the electron microscope images of at least 100 particles. Figure 2a–c shows the scanning electron microscopy (SEM) images of cobalt ferrites CF1, CF2, and CF3. The surface morphology admits the agglomerated coalescence behavior of the particles. This agglomeration shall be due to the interfacial surface tension phenomenon. The spinel nanoparticles consist of uniform cubic crystallites with the average size of 22.4 nm (CF1), 27.8 nm (CF2), and 31.3 nm (CF3) at temperatures of 400, 500, and 600 ∘C, respectively, in agreement with the XRD results. In this present study, the calcination temperature is considered the key factor for controlling the particle size of the final product of CoFe2O4 nanocrystals synthesized by thermal treatment method. The observed difference in particle size calculated by XRD and HR-SEM may be due to the molecular structural disorder and lattice strain, which results from the different ionic radii and/or clustering of the nanoparticles. Hence, the XRD method has a more stringent criterion and leads to smaller sizes. In Fig. 2, it is observed that both the obtained nanoparticles had sizes in the ranges 21–24 nm (CF1), 25–29 nm (CF2), and 30–33 nm (CF3). Actually, the mean particle size determined by SEM is very close to the average crystal size calculated by the Debye–Scherrer formula from the XRD pattern. This is in good agreement with the results of XRD analysis.
3.3 HR-TEM Analysis
A HR-TEM image of nanocrystalline CoFe2O4 is shown in Fig. 3a–c. The micrograph depicts the spherical morphology of the calcined nanocrystalline CoFe2O4 particles at 400, 500, and 600 ∘C (CF1, CF2, and CF3). The size of the nanoparticles increases with the heat treatment temperature, which may be evidenced from the effect of reflection broadening by comparing the (311) reflection of the samples. The average particle size of the CoFe2O4 calcined at 400 ∘C is 22 nm; at 500 ∘C, it grows to ca. 26 nm, and for the heat treatment at 600 ∘C, it is around 30 nm. These features could be explained because at higher substrate temperature the deposited radicals are characterized by higher surface kinetic energy, which permits them better accommodation and consequently produces a better-processed, denser material. It is seen that the size of clusters in the samples slightly increases with increasing calcination temperature [29, 30]. Only a small difference is observed in particle size as measured by XRD and SEM or TEM. The difference in the obtained values of the particle size of the CoFe2O4 nanomaterial is due to the fact that the SEM or TEM measurements are based on the difference between the visible grain boundaries, while XRD calculations measure the extended crystalline region that diffracts X-rays coherently. Hence, the XRD method has a more stringent criterion and leads to smaller sizes [31, 32].
3.4 PL Spectra
The optical properties of the cobalt ferrites were studied by means of luminescence spectroscopy. Since particle size is dependent on the heat treatment conditions, a shift in the photoluminescence (PL) emission spectra is expected to occur upon particle growth. In the emission spectra of CoFe2O4 (Fig. 4) prepared by the hydrothermal method, broad bands are observed and assigned to charge transfer between Fe3+ at octahedral sites and its surrounding O2− ions. In general, PL of CoFe2O4 shows strong emission bands in the UV (390 nm) region and blue (450 nm) and green (540 nm) emission bands in the visible region. The luminescence property of CoFe2O4 is a result of its spinel-type structure. In our study, with excitation at 365 nm, cobalt ferrite nanoparticles show the intrinsic emission peaks with their surrounding at 480–550 nm; the emission maximum in this work suggests such transition might be assisted by the intraband gap defects, such as oxygen vacancies. These defects provide donor levels near the conduction band edge of the oxide. The emission band position is highly dependent on the calcining temperature. It is confirmed that the surface morphology and the state of the synthesized nanoparticles are responsive and play an important role for the determination of PL spectra. A broad and strong peak in UV region and a weak band in the visible region confirmed the good crystallinity of the prepared nanoparticles [33–37].
3.5 VSM Measurements
Magnetic measurements of the samples were carried out at 300 K in order to identify the magnetic states and envisage their behavior. The VSM measurements were recorded in the −15 to + 15 kOe applied magnetic field range. The shape and area of the loops were found to depend strongly on the preparation method as well as on the ratio of the crystalline phases in the nanopowders. The shape of the hysteresis loop shows a characteristic weak ferromagnetic behavior. Both the samples displayed the normal (s-shaped) narrow hysteresis loops, and the magnetic parameters like saturation magnetization (Ms), remanent magnetization (Mr), and coercivity (Hc) of the cobalt ferrite samples (CF1, CF2, and CF3) were obtained.
Factors such as cation redistribution in the nanoregime at the A and B sites and surface defects shall lead to the change in the net magnetic moment resulting in such variation in the Ms values, also the variation in remanence magnetization observed for the samples in Fig. 5. CoFe2O4 nanoparticles attributed to the lattice defects magnetic super-exchange interaction from tetrahedral to octahedral sites and random magnetic orientation of spins on the cobalt ferrite nanoparticles. The low Hc and Mr confirm that the CoFe2O4 nanoparticles have a soft and weak ferromagnetic nature. The saturation magnetization (Ms), coercivity (Hc), and remanence (Mr) along with the average crystallite size of the three samples (CF1, CF2, and CF3) are summarized in Table 1.
Cobalt ferrites (CoFe2O4) have a normal spinel structure with ferromagnetic nature, due to the exchange between the ions occupying the tetrahedral and octahedral sites. The spinel ferrites with a cubic crystal structure have two different crystallographic sublattices for magnetic ions, tetrahedral (A) and octahedral (B) sublattices. In the case of spinel ferrites, the magnetic order mainly results from super-exchange interaction between the magnetic ions in the A and B sublattices mediated by oxygen ions. According to Neel’s two-sublattice model of ferrimagnetism, the magnetic moments of ions on the A and B sublattices are aligned anti-parallel to each other and their spins have a collinear structure. Therefore, total magnetization is M B– M A (M B and M A), the sum of the magnetic moments on the B sublattice and A sublattice, respectively. In the present study, cation distribution depends on the strength of the exchange interaction between the ions at the tetrahedral (A) and octahedral (B) sites. Co 2+ ions with zero magnetic moment have a strong preference to occupy the tetrahedral A sites, and Fe 3+ ions have a stronger preference to occupy the tetrahedral A sites as compared to the octahedral B sites. Also, it has zero net magnetization, because of the complete compensation of the sublattice magnetization. It is well known that the magnetic property of nanomaterials is strongly dependent on the shape and sizes of the particles and their crystallinity [38, 39]. However, the precursor is not completely decomposed at 400 ∘C and only those obtained at 500 ∘C are pure cobalt ferrite. On the other hand, at 600 ∘C, high temperature causes the VSM spectra to be much activated in order to decrease their other samples CF1 and CF3. From Table 1, Hc and Mr values of sample CF2 are higher than those of the other samples. The small difference in Ms, Mr, and Hc among the samples may be mainly related to the different temperatures and thus different shape anisotropies. This study illustrates the pertinence of the size and magnetic properties of CoFe2O4 samples, respectively. It is seen from these results that the value of coercivity decreases with increasing particle size. This variation of Hc with particle size can be explained on the basis of domain structure, critical diameter, and the anisotropy of the crystal. A crystallite will spontaneously break up into a number of domains in order to reduce the large magnetization energy it would have if it were a single domain [40].
3.6 N 2 Adsorption/Desorption Isotherms
From the nitrogen adsorption/desorption isotherm values at 83 K of the nickel aluminate, which the specific surface areas (SBET) together with the pore radius (Rp) and pore volume (Vp) are calculated, are given in Table 2. The samples are heated all over and sintered uniformly, and then grains of the samples are fine and homogeneous, which are in favor of improving the catalytic properties.
The N 2 adsorption–desorption isotherms of all the samples are type IV at high relative pressures (0.4 < p/p0 < 0.8) because capillary condensation under N 2 presents an H1 hysteresis loop (Fig. 6). The adsorption and desorption branches across a narrow range of pressures displayed clear parallel trends. However, with increasing temperature, the cobalt ferrite samples showed a wide range of relatively flat pressure hysteresis loops, and the adsorption and desorption branches closed at slightly lower pressures. According to Kelvin’s law, the low pressure of capillary condensation when the aperture size decreased indicated that the surface area increases. The specific surface area and pore volume decrease as a result of temperature increases in cobalt ferrite nanoparticles. The surface area parameters of the samples are varied according to the preparation method. Therefore, it is believed that the high surface area of sample CF2 could enhance the catalytic properties than that of samples CF1 and CF3. Surface and pore properties of the CoFe2O4 particles calcined at different temperatures are presented in Table 2. The data concerning pore size indicate that the pore size increases significantly with the reaction time, although the total pore volume was not practically altered. High surface areas were obtained for the samples calcined at 500 ∘C for CF2 and also for both samples calcined at 400 ∘C (CF1) and 600 ∘C (CF3). It was also observed that the surface area significantly decreases at higher calcination temperatures [41–43]. In the SEM figures, pores could be identified between the nanoparticles. These types of catalysts with high surface areas having narrow distributions of particles can be prepared by this soft chemical method. The compound prepared with such an excellent surface area may be promising for application as a catalyst [44, 45].
3.7 Catalytic Oxidation of Benzyl Alcohol
The catalytic oxidation of benzyl alcohol was studied using cobalt ferrite nanoparticles to investigate its catalytic performance. 0.3 g of as-prepared samples (CF1, CF2, and CF3) along with H2O2 as oxidant (5 mmol) in acetonitrile medium was taken in a batch reactor, and the contents were heated at 50 ∘C for 6 h. When the oxidized product was analyzed using gas chromatography (GC), it showed the conversion and selectivity of the benzaldehyde. It was noticed that the nanocobalt ferrites possessed a higher yield with good selectivity for benzaldehyde. The conversion percentage along with selectivity is given in Table 3. Generally, solvent plays an important role in the conversion and product distribution of oxidation reactions. Acetonitrile readily dissolves in H2O2 along with benzyl alcohol as it is polar and having a polarity index of 5.8. This facilitates the adsorption of reactants on the catalyst surface and increases the efficiency of the conversion and selectivity. Solvent acetonitrile can activate peroxide by forming a perhydroxyl anion (OOH −) that nucleophilically attacks the nitrile to generate a peroxycarboximidic acid intermediate, which is a good oxygen transfer agent. Both organic substrate and the oxidant H2O2 dissolve in acetonitrile, forming a uniform solution. In addition, acetonitrile has a comparatively good solubility power for both the organic substrate and the liquid-phase H2O2. This catalytic system in the polar solvents such as acetonitrile gives better conversion results [46–48]. In addition, H2O2 is proven to be an efficient and environmentally friendly oxidant, since the product is only aldehyde [49].
4 Conclusion
The effect of the hydrothermal method and calcination temperature on the structural, morphological, magneto-optical, and catalytic activity for the selective oxidation of benzylic alcohols was investigated. The method used in this study is found to be of great advantage to prepare multicomponent magnetic ferrite materials possessing desirable catalytic activity. In conclusion, a high surface area of cobalt ferrite with variable catalytic activity can be achieved with a lower crystal size. The synthesized magnetic nanocatalyst provides an efficient and environment-friendly method for oxidation of aromatic alcohols to corresponding carbonyls with a high yield. Hence, this catalyst is a viable alternative for oxidation of alcohols.
References
Stewart, S.J., Tueros, M.J., Cernicchiaro, G., Scorzelli, R.B. Solid. State. Commun. 129, 347 (2004)
Manikandan, A., Hema, E., Durka, M., Seevakan, K., Alagesan, T., Arul Antony, S.: J. Supercond. Nov. Magn. 28, 1783 (2015)
Hema, E., Manikandan, A., Karthika, P., Durka, M., Arul Antony, S., Venkatraman, B.R.: J. Supercond. Nov. Magn. 28, 2539 (2015)
Manikandan, A., Durka, M., Arul Antony, S.: J. Supercond. Nov. Magn. 28, 2047 (2015)
Pui, A., Gherca, D., Cornei, N.: Mater. Res. Bull. 48, 1357 (2013)
Manikandan, A., Durka, M., Arul Antony, S.: Adv. Sci. Eng. Med. 7, 33 (2015)
Manikandan, A., Kennedy, L.J., Bououdina, M., Vijaya, J.J.: Ceram. Int. 39, 5909 (2013)
Manikandan, A., John Kennedy, L., Bououdina, M., Judith Vijaya, J.: J. Magn. Magn. Mater. 249, 349 (2014)
Manikandan, A., Vijaya, J.J., Sundararajan, M., Meganathan, C., Kennedy, L.J., Bououdina, M.: Superlattices and Microstructures 64, 118 (2013)
Blanco-Gutiérrez, V., Gallastegui, J.A., Bonville, P., Torralvo-Fernández, M.J., Saez-Puche, R.: J. Phys. Chem. C 116, 24331 (2012)
Martínez, B., Obradors, X.: Phys. Rev. Lett. 181, 80 (1998)
Topkaya, R., Akman, Ö., Kazan, S., Aktas, B., Durmus, Z., Baykal, A.: J. Nanopart. Res. 14, 1156 (2012)
Kumar, S., Singh, V., Aggarwal, S., Mandal, U.K., Kotnala, R.K.: J. Phys. Chem. C 114, 6272 (2010)
Sertkol, M., Köseoglu, Y., Baykal, A., Kavas, H., Baskaran, A.C.: J. Magn. Magn. Mater. 321, 157 (2009)
Manikandan, A., Durka, M., Arul Antony, S.: J. Supercond. Nov. Magn. 27, 2841 (2014)
Hema, E., Manikandan, A., Karthika, P., Antony, S.A., Venkatraman, B.R.: J. Supercond. Nov. Magn. 28, 2539 (2015)
Manikandan, A., Durka, M., Arul Antony, S.: J. Supercond. Nov. Magn. 28, 209 (2015)
Manikandan, A., Durka, M., Arul Antony, S.: J. Supercond. Nov. Magn. 28, 1405 (2015)
Narayanan, S., Vijaya, J.J., Sivasanker, S., Ragupathi, C., Sankaranarayanan, T.M., Kennedy, L.J.: J. Porous Mater. doi:10.1007/s10934-016-0129-8
Sajjia, M., Oubaha, M., Prescott, T., Olabi, A.G.: J. Alloys Compd. 506, 400 (2010)
Ragupathi, C., Vijaya, J.J.: Kennedy, Adv. Powder Technol. 267, 25 (2014)
Manikandan, A., Arul Antony, S., Sridhar, R., Bououdina, M.: J. Nanosci. Nanotechnol. 15, 4948 (2015)
Manimegalai, D.K., Manikandan, A., Moortheswaran, S., Arul Antony, S.: J. Supercond. Nov. Magn. 28, 2755 (2015)
Manikandan, A., Hema, E., Durka, M., Amutha Selvi, M., Alagesan, T., Arul Antony, S.: J. Inorg. Organomet. Polym. 25, 804 (2015)
Valan, M.F., Manikandan, A., Arul Antony, S.: J. Nanosci. Nanotechnol. 15, 4543 (2015)
Demortiere, A., Panissod, P., Pichon, B.P., Pourroy, G.D., Guillon, Donnio, B.: Nanoscale 3, 225 (2011)
Kumar, V., Rana, A., Kumar, N., Pant, R.P.: Int. J. Appl. Ceram. Technol. 8, 120 (2011)
El-Okr, M.M., Salem, M.A., Salim, M.S., El-Okr, R.M., Ashousha, M., Talaat, H.M.: J. Magn. Magn. Mater. 920, 323 (2011)
Manikandan, A., John Kennedy, L., Judith Vijaya, J.: vol. 49, p 117 (2013)
Chinnaraj, K., Manikandan, A., Ramu, P., Arul Antony, S., Neeraja, P.: J. Supercond. Nov. Magn. 28, 179 (2015)
Manikandan, A., Judith Vijaya, J., John Kennedy, L., Bououdina, M.: J. Mol. Struct. 1035, 332 (2013)
Manikandan, A., Durka, M., Arul Antony, S., Inorg, J.: Organomet. Polym. 25, 1019 (2015)
Ragupathi, C., Vijaya, J.J., Narayanan, S., Jesudoss, S.K., Kennedy, L. J.: Ceram. Int. 41, 2069 (2014)
Ragupathi, C., Vijaya, J.J., Kennedy, L.J.: Ceram. Bououdina Int. 40, 13067 (2014)
Ragupathi, C., Vijaya, J.J., Kennedy, L.J.: J. Saudi. Chem. Soc. (2014). (article in press). doi:10.1016/j.jscs.2014.01.006
Alison, A, de Souza Goncalves, A., Davolos, M.R.: J. Sol–Gel Sci. Technol. 49, 101 (2009)
Ragupathi, C., Vijaya, J.J., Surendhar, P., Kennedy, L.J.: Polyhedron 72, 7 (2014)
Neel, L., Acad, C.R.: Sci. Paris 230, 375 (1950)
Ragupathi, C., Vijaya, J.J., Kennedy, L.J., Bououdina, M.: Mater Sci. Semicond. Process 24, 146 (2014)
Kinemuchi, Y., Ishizaka, K., Suematsu, H., Jiang, W., Yatsui, K.: Thin Solid Films 407, 109 (2002)
Manikandan, A., John Kennedy, L., Arul Mary, J., Dinesh, A., Judith Vijaya, J.: J. Ind. Eng. Chem. 20, 2077 (2014)
Manikandan, A., John Kennedy, L., Judith Vijaya, J.: J. Nanosci. Nanotechnol. 13, 2986 (2013)
Manikandan, A., Saravanan, A., Arul Antony, S., Bououdina, M.: J. Nanosci. Nanotechnol. 15, 4358 (2015)
Yang, C.M., Liu, P.H., Ho, Y.F., Chiu, C.Y., Chao, K.J. Chem. Mater. 15, 275 (2003)
Manikandan, A., Sridhar, R., Arul Antony, S., Ramakrishna, S.: J. Mol. Struct. 1076, 188 (2014)
Jayasree, S., Manikandan, A., Arul Antony, S., Uduman Mohideen, A.M., Barathiraja, C.: J. Supercond. Nov. Magn. 29, 253 (2016)
Jayasree, S., Manikandan, A., Uduman Mohideen, A.M., Barathiraja, C., Arul Antony, S.: Adv. Sci. Eng. Med. 7, 672 (2015)
Hema, E., Manikandan, A., Gayathri, M., Durka, M., Arul Antony, S., Venkatraman, B.R.: J. Nanosci. Nanotechnol. 16, 5929 (2016)
Hai, Z., Ying, L., Xungao, Z.: Chin. J. Catal. 32, 1693 (2011)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paramasivan, P., Venkatesh, P. A Novel Approach: Hydrothermal Method of Fine Stabilized Superparamagnetics of Cobalt Ferrite (CoFe2O4) Nanoparticles. J Supercond Nov Magn 29, 2805–2811 (2016). https://doi.org/10.1007/s10948-016-3586-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-016-3586-z