Abstract
CuAlO2 thin films were prepared on quartz glass and sapphire substrates by chemical solution deposition method using copper acetate monohydrate, aluminum nitrate nonahydrate and 2-methoxyethanol as starting precursor and solvent. The effects of annealing temperature on the structural, morphological, electrical and optical properties have been studied. Via the optimized annealing treatment condition, CuAlO2 film annealed at 850 °C in nitrogen flow of 400sccm under atmosphere pressure exhibits the best performance with the lowest room temperature resistivity of 3.6 × 102 Ω cm and the highest optical transmission in the visible region (>70% at around 600 nm wavelength). CuAl2O4 and CuO phases, not CuAlO2 phase are obtained when annealing temperature is lower than 850 °C. However, a further increase of annealing temperature weakens the crystallization quality and deteriorates the surface morphology of CuAlO2 films as the annealing temperature exceeds 850 °C, leading to an increase in the resistivity and a decrease of the optical transmission in the visible region of CuAlO2 films.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Transparent conducting oxides (TCOs) play a key role in a number of thin film optoelectronic devices including liquid crystal displays, organic light-emitting diodes, and solar cells [1–3], owing to their unique features of optical transparency in the visible region and controllable electrical conductivity. However, almost all of the well-known TCOs such as Al-doped ZnO, Sn-doped In2O3 and F-doped SnO2 have n-type conductivity, the lack of p-type TCOs severely limits the potential applications of these materials, because many of the active functions in semiconductors originate from the nature of the p-n junction.
In 1997, Kawazoe et al. [4] first reported CuAlO2 thin film as a candidate of p-type TCOs. This discovery has opened up the possibility of fabricating p-n junctions using p- and n-type TCOs. CuAlO2 has a layered delafossite structure with the rhombohedral space group of \( R\mathop 3\limits^{\_} m \). It is composed of an alternate stacking of an O–Cu–O dumbbell layer and an AlO6 edge-sharing octahedra layer (shown in Fig. 1).
So far, various deposition techniques have been employed to fabricate CuAlO2 thin films, for instance, pulsed laser deposition [4, 5], sputtering [6–9, 15], chemical vapor deposition [10, 11], spray pyrolysis [12], e-beam evaporation [13], and so on. Chemical solution deposition (CSD) as a very useful method has been widely used for the preparation of thin films of different materials and devices, which is considered as a facile route for a large-area coating at relatively low temperature and low cost. However, there are few reports about the synthesis of CuAlO2 thin films via CSD method. Tonooka et al. [14] had succeeded in obtaining CuAlO2 films by way of solution method, but there was simultaneously some impurity in the product, such as CuO and CuAl2O4.
It is well known that the characteristics of CSD derived thin films are generally affected by the preparation conditions such as the starting materials, sol concentration, the thermal treatment temperature, types of substrates, and so on. All these factors have distinct effects on the behaviors of thin films. In this work, we have investigated the influence of annealing treatment on the structural, morphological, electrical and optical properties for CSD derived CuAlO2 thin films.
2 Experimental procedure
CuAlO2 thin films were prepared by CSD method. Copper acetate monohydrate (Cu(CH3COO)2·H2O) and aluminum nitrate nonahydrate (Al(NO3)3·9H2O) with [Cu]/[Al] molar ratio of 1.0 were first dissolved in 2-methoxyethanol and the sol concentration was 0.2 M. The solution was stirred at room temperature for more than 5 h in order to get a well mixed precursor solution.
Before deposition, the substrates were cleaned with acetone, ethanol and de-ionized water successively in ultrasonic bath. The films were deposited by spin coating route with a rotating speed of 6,000 rpm for 20 s. After each coating, the as-deposited films were dried in air at 400 °C for 20 min to evaporate the solvent and remove organic residuals. The procedures from coating to drying were repeated eight times until the desired thickness of the sintered films was reached. Finally, the films were inserted to a tube furnace and annealed at 650–1,050 °C for 3 h. A warming temperature speed was 10 °C/min and a natural cooling was maintained after finishing the annealing.
X-ray diffraction (XRD) using a Philips X’pert Pro diffractometer with Cu K α radiation was carried out to check up the crystalline structure. The surface morphology and microstructure of the films were characterized by field-emission scanning electronic microscopy (FE-SEM, FEI Sirion 200 type). The electrical resistance was obtained using a physical properties measurement system (PPMS, Quantum-designed) with a standard four-point probe method in the four-probe steady state mode. The electrical resistivity was obtained from the mathematical product of resistance and thickness. Optical transmittance of the derived films was measured using automated scanning monochromator (Varian-designed Cary-5E type). The measurement was performed using air as reference in a wavelength range of 200–1,200 nm.
3 Results and discussion
Figure 2 shows XRD patterns of thin films annealed at 850 °C in air [(a) in figure] and different nitrogen flow under atmosphere pressure [(b), (c) and (d) in figure] on quartz glass [(a), (b) and (c) in figure] and sapphire [(d) in figure] substrates. According to Fig. 2c and d, thin films annealed in nitrogen flow of 400sccm, regardless of the substrate material, exhibit only CuAlO2 XRD peaks, while thin film annealed in air exhibits no CuAlO2 peaks, but CuAl2O4 and CuO peaks, as shown in Fig. 2a. These imply that O2 partial pressure may be a key factor for the preparation of CuAlO2 thin film. In order to support this conclusion, we have prepared another thin film annealed in nitrogen flow of 100sccm (Fig. 2b), in which we observed CuAl2O4 and CuO peaks in addition to CuAlO2 peaks. From the phase diagram of Cu–Al–O material [16], it is clear that CuAlO2 phase exists between 1,000 °C and 1,250 °C at p(O2) = 0.21 atm. Below 1,000 °C, CuAl2O4 and CuO are the stable phases. Therefore, in air Cu2+ ions are the more stable when thin film is annealed. Even if CuAlO2 phase was synthesized, it also can be decomposed into a mixture of CuAl2O4 and CuO by the following reaction:
On the basis of another Cu–Al–O phase diagram [17], CuAlO2 phase seems to be stable at lower temperature and O2 partial pressure. Moreover, the reaction of CuAlO2 and O2 can be hindered using a protective atmosphere, such as N2, to avoid the oxidation. A similar behavior has been reported by Lan et al. [15]. They found that CuAlO2 could be oxidized into CuAl2O4 and CuO when the sputtered film was annealed at 900 °C in air. In comparison with the annealed films on quartz glass substrates, the crystallization quality of that on sapphire substrate raises. This phenomenon is related to the lattice match between the film and the substrate [18]. However, CuAlO2 diffraction peaks (101), (012), (104), (107), (018), (110) and (1,010) were still observed even on the (0,001) sapphire substrate.
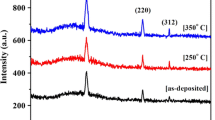
Figure 3 shows the XRD patterns of thin films on sapphire substrates with different annealing temperatures in nitrogen flow under atmosphere pressure. As seen from Fig. 3, even though thin films are annealed in nitrogen flow of 400sccm, not CuAlO2 peaks, but CuAl2O4 and CuO peaks appear when the annealing temperature is lower than 850 °C. According to Cu–Al–O phase diagram [17], it can be deduced that in order to get pure CuAlO2 phase, the lower O2 partial pressure, i.e., the more nitrogen flow is needed as the annealing temperature decreases. From Fig. 3, we also observed that pure CuAlO2 films are obtained and the crystallization quality of CuAlO2 decreases with the increase of the annealing temperature when the annealing temperature exceeds 850 °C. In general, the crystallinity of thin film should be improved with increase in annealing temperature, but the weakened crystallinity of our films may be due to the poor morphology induced by quick rearrangement and coalescence of CuAlO2 grains in the film at high temperature [15], which can be seen later from the FESEM images.
Figure 4 shows the FESEM micrographs of CuAlO2 films on sapphire substrates annealed at different temperatures in nitrogen flow under atmosphere pressure. A strong influence of annealing temperature on the grain growth can be clearly observed. It can be seen from Fig. 4a that CuAlO2 film annealed at 850 °C shows homogeneous surface covered by many small grains, and inter-grain connection is developed. Increasing the annealing temperature the surface morphology of CuAlO2 films begin to deteriorate. A few virgate grains appear in the surface of CuAlO2 films annealed at 950 and 1,050 °C, as shown in Fig. 4b and c. This result can be attributed to the agglomeration of small grains, which is induced by the diffusion of the atoms with enough thermal energy at higher temperature [19]. Meanwhile, there also exist some grain boundary macro-pores in the CuAlO2 films (Fig. 4b and c). From Fig. 4c, some protuberances are distributed in the surface of the film annealed at 1,050 °C. Jiang et al. [20] also found the same phenomenon that they have attributed to the extrusion caused by volume expansion at higher annealing temperature. The degradation of surface morphology, in accordance with the results of XRD measurement, supports the decrease of crystallinity for the film annealed at higher temperature. The cross sectional micrograph of CuAlO2 film annealed at 850 °C is shown in Fig. 4d. Though the annealing temperature is different, it is considered that the thicknesses of all the films are approximately 270 nm owing to the same preparation conditions. The thickness of the derived films is reproducible, and the thickness can be controlled by tuning the solution concentration.
Figure 5 shows the temperature dependence of the resistivity for CuAlO2 thin films on sapphire substrates with different annealing temperature in nitrogen flow of 400sccm under atmosphere pressure. It is immediately evident that there is semiconductive temperature dependence for all the films. From Fig. 5, we can see that the resistivity of CuAlO2 films at room temperature exhibits an increase with increasing annealing temperature and the film annealed at 850 °C demonstrates the minimum resistivity value of 3.6 × 102 Ω cm. Though the number of charge carriers should increase as a result of thermal excitation, the degenerating morphology, as shown in Fig. 4, gives rise to the great decrease in the mobility of carriers and finally leads to the increase of the resistivity when the annealing temperature increases. Lan et al. [15] also reported CuAlO2 films with higher resistivity, which were deposited by rf magnetron sputtering. However, Kawazoe et al. [4] achieved a CuAlO2 film via pulsed laser deposition, having a larger room temperature conductivity up to 1Scm−1. These different results may be related to the preparation technology.
To further study the intrinsic characteristics of CuAlO2 film, the Arrhenius plot of logσ versus 1,000/T is shown in Fig. 6. The linear dependence relationship in the temperature range of 250–300 K indicates that CuAlO2 films are thermally activated semiconductors. The activation energy values are obtained from the slopes by best fitting as 0.108, 0.140 and 0.128 eV for the films annealed at 850, 950 and 1,050 °C, respectively, which are far smaller than half of the bandgap. This might be a suggestion of hole transparent in the valence band due to the positive sign of the Seebeck coefficient [4, 21]. In comparsion to the result of Yanagi et al. [5], the activation energy of our films is smaller, yet the resistivity is larger because of the worse morphology.
Figure 7 shows the optical transmission of CuAlO2 films on sapphire substrates with different annealing temperatures in nitrogen flow of 400sccm under atmosphere pressure. It is obvious that the average transmission of the film annealed at 850 °C is high (>70% at around 600 nm wavelength) and a sharp absorption edge is observed around the wavelength 400 nm, which is an indication of the good crystallinity of the film. In comparison with films prepared by pulsed laser deposition [4], sputtering [8, 22] and MOCVD [11], the optical transmission of our film is relatively high. As seen in Fig. 7, the transmission in the visible region decreases and the absorption edge shifts to the long wavelength as a result of the increased annealing temperature. The decrease of the crystalline quality and the poor morphology may be the reasons for this reduction. In fact, as a result of the increase of the density of defect states in the films, the light scattered increases, lowering the optical transmission [23].
In order to show the effect of the annealing temperature on the optical bandgap of the films, the absorption coefficient (α) was calculated using Manifacier model [24]. The dependence of α on the photon energy (hν) was fitted to the relation [25]:
where A is a constant, E g is the optical bandgap and the exponent n depends on the type of the transition. For direct allowed transition, indirect allowed transition and direct forbidden transition, n is 1/2, 2 and 3/2, respectively. The plot of (αhν)2 versus hν of the films deposited at different annealing temperature is shown in Fig. 8. Extrapolation of the linear portion of the plots of (αhν)2 versus hν to α = 0 gives the optical bandgap of CuAlO2 films for direct allowed transition. The optical bandgap decreases from 3.23 to 3.00 eV with increasing the annealing temperature from 850 to 1,050 °C, which results from the arising of defect states induced by the decrease of the crystallinity. Our films show narrower optical bandgap than some previously reported [4, 8, 13]. A lot of study have been done on the band-structure calculation of CuAlO2 in the last few years [17, 26]. All the cases suggest that CuAlO2 band edges are very complicated, and the optical bandgap relies heavily on the preparation technique of CuAlO2 film. Therefore, it is hard to determine the origin of these optical bandgap of our films. A more powerful research is needed in the future study.
4 Conclusion
CuAlO2 films were successfully deposited on quartz glass and sapphire substrates by chemical solution deposition method. In this study, the influences of annealing temperature on the structural, morphology, electrical and optical characteristics have been investigated. It is observed that the preparation of pure CuAlO2 film is closely related to the annealing temperature and O2 partial pressure. CuAlO2 film annealed at 850 °C in nitrogen flow of 400sccm under atmosphere pressure has the highest optical transmission (>70% at around 600 nm wavelength) and the lowest resistivity (3.6 × 102 Ω cm at room temperature). The resistivity increases and the optical transmission in the visible region decreases as the annealing temperature increases from 850 to 1,050 °C, largely due to the decline of the crystallinity and the degraded morphology. CuAlO2 films can be described by the thermal-activation theory in the temperature range of 250–300 K. The activation energy and the optical bandgap of our films are lower in comparison with the previously reported, owing to the different preparation methods.
References
Drevillon B, Kumar S, Cabarrocas PRi, Siefert JM (1989) Appl Phys Lett 54:2088
Emziane M, Durose K, Halliday DP, Bosio A, Romeo N (2005) Appl Phys Lett 87:251913
Yang Y, Wang L, Yan H, Jin S, Marks TJ, Li SY (2006) Appl Phys Lett 89:051116
Kawazoe H, Yasukawa M, Hyodo H, Kurita M, Yanagi H, Hosono H (1997) Nature 389:939
Yanagi H, Inoue S, Ueda K, Kawazoe H, Hosono H, Hamada N (2000) J Appl Phys 88:4159
Banerjee AN, Maity R, Chattopadhyay KK (2003) Mater Lett 58:10
Dong GB, Zhang M, Zhao XP, Li YC, Yan H (2009) J Cryst Growth 311:1256
Tsuboi N, Moriya T, Kobayashi S, Shimizu H, Kato K, Kaneko F (2008) Jpn J Appl Phys 47:592
Banerjee AN, Chattopadhyay KK (2005) J Appl Phys 97:084308
Gong H, Wang Y, Luo Y (2000) Appl Phys Lett 76:3959
Wang Y, Gong H (2000) Chem Vap Deposition 6:285
Bouzidi C, Bouzouita H, Timoumi A, Rezig B (2005) Mater Sci Eng B 118:259
Kim DS, Park SJ, Jeong EK, Lee HK, Choi SY (2007) Thin Solid Films 515:5103
Tonooka K, Shimokawa K, Nishimura O (2002) Thin Solid Films 411:129
Lan W, Zhang M, Dong GB, Wang YY, Yan H (2007) J Mater Res 22:3338
Jacob KT, Alcock CB (1975) J Am Ceram Soc 58:192
Ingram BJ, Mason TO, Asahi R, Park KT, Freeman AJ (2001) Phys Rev B 64:155114
Shy JH, Tseng BH (2005) J Phys Chem Solids 66:2123
Thompson CV (1990) Annu Rev Mater Sci 20:245
Jiang HF, Lei HC, Zhu XB, Li G, Yang ZR, Song WH, Dai JM, Sun YP, Fu YK, (in press) J Alloys Compd
Lee MS, Kim TY, Kim D (2001) Appl Phys Lett 79:2028
Lan W, Cao WL, Zhang M, Liu XQ, Wang YY, Xie EQ, Yan H (2009) J Mater Sci 44:1594
Reddy AS, Reddy PS, Uthanna S, Rao GM (2006) J Mater Sci Mater Electron 17:615
Manifacier JC, Gasiot J, Fillard JP (1976) J Phys E Sci Instrum 9:1002
Ziegler E, Heinrich A, Oppermann H, Stover G (1981) Phys Status Solidi A 66:635
Nie X, Wei SH, Zhang SB (2002) Phys Rev Lett 88:066405
Acknowledgments
This work was supported by the National Key Basic Research under contract no. 2007CB925002, and the National Nature Science Foundation of China under contract no. 10774146, 50802096, Anhui Province NSF Grant No. 070414162, and Director’s Fund of Hefei Institutes of Physical Science, Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, G., Zhu, X., Lei, H. et al. Preparation and characterization of CuAlO2 transparent thin films prepared by chemical solution deposition method. J Sol-Gel Sci Technol 53, 641–646 (2010). https://doi.org/10.1007/s10971-009-2143-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-009-2143-7