Abstract
Thin films of copper aluminum oxide (CuAlO2) were prepared on glass substrates by dc magnetron sputtering at a substrate temperature of 523 K under various oxygen partial pressures in the range 1 × 10−4–3 × 10−3 mbar. The dependence of cathode potential on the oxygen partial pressure was explained in terms of oxidation of the sputtering target. The influence of oxygen partial pressure on the structural, electrical and optical properties was systematically studied. p-Type CuAlO2 films with polycrystalline nature, electrical resistivity of 3.1 Ω cm, Hall mobility of 13.1 cm2 V−1 s−1 and optical band gap of 3.54 eV were obtained at an oxygen partial pressure of 6 × 10−4 mbar.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transparent conducting oxides (TCO) are materials, which exhibit high electrical conductivity and high optical transmittance in the visible spectral range. Electron doped ZnO, In2O3, SnO2 are widely used as transparent electrodes in flat panel displays, solar cells and touch panels [1]. Realization of p-type transparent conducting oxides is a milestone to expand the utilization of these layers as transparent semiconductors because a wide variety of active functions of semiconductor elements comes from p–n junctions. Combination of two types of TCOs in the form of p–n junctions leads to a functional window, that transmits the visible portion of solar radiation and generate electricity by the absorption of ultraviolet radiation [2]. Recently wide band gap p-type TCOs such as CuAlO2 [2], LaCuOS and LaCuOSe [3], CuGaO2 [4] and SrCu2O2 [5] films have been produced successfully by various techniques with optimized electrical and optical properties. Inherent difficulty in producing p-type TCOs films is the strong localization of holes with in the metal oxides due to the electronegative character of oxygen ions. Metal defects or excess oxygen ions are supposed to induce holes within the materials and their deviations from the stoichiometric composition of the components can be done by controlling the preparation conditions of the materials. The enhancement of p-type conductivity of these materials can be achieved in the presence of oxygen atmosphere during deposition process and post deposition annealing [6, 7]. Thomas [8] suggested that the chemical formula for this material would be CuAlO2+x with x as low as 0.001 at% of excess oxygen (x = 5 × 10−5) over the stoichiometric value with in the films. Various thin film deposition techniques such as pulsed laser deposition [2, 9], chemical vapour deposition [6, 10], sol–gel process [11], dip coating [12], spin-on technique [13], dc sputtering [14, 15] and rf sputtering [7, 16, 17] were employed for preparation of highly conducting CuAlO2 thin films. In literature, it was reported that CuAlO2 films were successfully deposited by pulsed laser deposition [2] on small area substrates and is not suitable for large-scale industrial production. In the semiconductor industry, the most widely used thin film deposition techniques for large area production are sputtering and chemical vapour deposition. These techniques allow the production of uniform films on large area substrates and hence are suitable for mass production of integrated circuit devices. Among the sputtering techniques, dc magnetron sputtering is one of the best techniques for preparation of films on large area substrates with required chemical composition at low substrate temperatures. The physical properties of the films formed by dc magnetron sputtering mainly depend on the process parameters such as sputtering power, sputtering pressure, oxygen partial pressure, substrate bias voltage and substrate temperature. In this investigation we prepared the CuAlO2 films by dc magnetron sputtering under various oxygen partial pressures and studied the influence of the oxygen partial pressure on the structural, electrical and optical properties.
Experimental
The CuAlO2 films were deposited on glass substrates by dc reactive magnetron sputtering using the copper–aluminum alloy (in the ratio 1:3 due to the difference in the sputter yield) target. The sputtering system is capable of producing the base pressure of 5 × 10−6 mbar. The sputter chamber was pumped with diffusion pump and rotary pump combination. The pressure in the sputter chamber was measured using digital Pirani and Penning gauge combination. A circular planar magnetron of 100 mm diameter was used as the sputter cathode. The magnetron target assembly was mounted on the top plate of the sputter chamber such that the sputtering could be done with sputter down configuration. A continuously variable dc power supply of 750 Volts and 3 Amps was used for sputtering. Pure argon was used as sputter gas and oxygen as reactive gas. The flow rates of both argon and oxygen gases were controlled individually by Tylan mass flow controllers. The sputtering conditions maintained during the growth of CuAlO2 films are given in Table 1. The deposited films were characterized by studying crystallographic structure, electrical and optical properties. The crystallographic structure of the films was analyzed using X-ray diffraction. Copper K α radiation with wavelength of 0.15416 nm was used as X-ray source. X-ray diffraction profiles were recorded in 2θ range 10–70°. The electrical resistivity and Hall mobility were studied by employing van der Pauw method [18]. The optical transmittance (T) and reflectance (R) of the films was recorded using Hitachi U-3400 UV–Vis–NIR double beam spectrophotometer in order to determine the optical absorption and energy band gap. The thickness of the films used in this study is in the range of 120–130 nm.
Results and discussion
Glow discharge characteristics of Cu–Al alloy target
Figure 1 shows the variation of the cathode potential (copper–aluminum alloy target) with the oxygen partial pressure for different cathode currents. At a cathode current, the cathode potential initially showed a gradual increase with the increase of oxygen partial pressure. At a critical oxygen partial pressure the cathode potential starts decreasing and thereafter increased again at higher oxygen partial pressures. At a constant cathode current of 250 mA, the initial increase of cathode potential from 384 to 394 V with the increase of oxygen partial pressure from 5 × 10−5 mbar to 1 × 10−4 mbar can be explained interms of formation negative oxygen ions. As the oxygen partial pressure increased from 1 × 10−4 to 6 × 10−4 mbar the cathode potential decreased from 394 to 292 V. When the oxygen partial pressure increased the target poisoning resulted in the formation of oxide layer on the target surface. Since oxide has higher secondary electron emission coefficient than that of metal, the total number of electrons as well as ionization increased, leading to the decrease of cathode potential with increase of oxygen partial pressure. Further increase in the oxygen partial pressure to 3 × 10−3 mbar, the cathode potential increased to 324 V. This was due to the increase in the thickness of oxide layer formed on the surface of the target which causes a decrease in the electrical conductivity of the sputtering target. Therefore higher cathode potential is needed to break this insulating barrier [19], resulting in the increase of cathode potential. Such a dependence of cathode potential on the oxygen partial pressure was earlier observed in the sputtering of aluminum [20].
Figure 2 shows the variation of deposition rate of CuAlO2 films with the oxygen partial pressure. The deposition rate of the films is directly influenced by the oxygen partial pressure maintained in the sputter chamber during the preparation of the films. The deposition rate of the films formed at low oxygen partial pressure of 1 × 10−4 mbar was 27.3 nm min−1. As the oxygen partial pressure increased to 1 × 10−3 mbar, the deposition rate decreased to 6.6 nm min−1 and at higher oxygen pressures it remained almost constant. The decrease in deposition rate with increase of oxygen partial pressure is due to the oxidation of the sputter target hence of low sputtering yield [21]. It can be seen that the oxygen partial pressure at which the cathode potential starts decreasing corresponds to the drop in the deposition rate of the films. Oxide films are generally prepared in the transition region where the deposition rate starts reducing.
Structural properties of CuAlO2 films
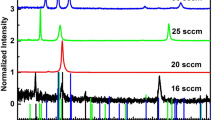
Figure 3 shows the X-ray diffraction spectra of CuAlO2 films formed at different oxygen partial pressures. The diffraction profiles indicated that the films were polycrystalline in nature. The films formed at an oxygen partial pressures 2 × 10−4 mbar showed two peaks at 2θ of 31.53° and 36.34° corresponding to the presence of (006) planes of CuAlO2 and (111) planes of Cu2O. When the oxygen partial pressure increased to 6 × 10−4 mbar the peak related to the Cu2O disappeared and additional peaks observed at 2θ = 15.61°, 37.66°, 48.29° and 65.51° identified as (003), (012), (009) and (110) along with (006) planes of CuAlO2. It indicates that the films were of Rhombohedral structure with space group of R3m and is in good agreement with the standard data [JCPDS. No 09-0185]. The intensity of the peaks increased with the increase of oxygen partial pressure. The films formed at higher oxygen partial pressures (>6 × 10−4 mbar) exhibited an additional peak at 2θ = 25.65° related to the (012) plane of Al2O3 phase along with CuAlO2. It was also observed that the intensity of peaks decreased with the increase of oxygen partial pressure and disappeared the (006) and (104) plane of CuAlO2. From these studies it revealed that single phase films of CuAlO2 were obtained at an oxygen partial pressure of 6 × 10−4 mbar. The grain size (L) of the films was evaluated from the full width half maximum intensity of X-ray diffraction peak of (006) using the Scherrer’s relation [22].
where k is a constant with a value of 0.89 for Cu target, β the half intensity width of the peak measured in radians. The grain size of the films increased from 10 nm to 35 nm with the increase of oxygen partial pressure from 2 × 10−4 mbar to 6 × 10−4 mbar beyond which it decreased to 15 nm at higher oxygen partial pressure of 1 × 10−3 mbar. The increase in grain size with the increase of oxygen partial pressure upto 6 × 10−4 mbar was due to the improvement in the crystallinity of the films. The decrease in the grain size at oxygen partial pressures >6 × 10−4 mbar may be due to the presence of Al2O3. It was also reported that higher the Al2O3 content leads to amorphous nature of the films. The grain size of the films obtained was comparable to metal organic chemical vapour deposited films [6] and smaller than the pulsed laser deposited films [2].
Electrical properties
The electrical properties of the films were highly influenced by the oxygen partial pressure. Figure 4 shows the electrical resistivity (ρ), Hall mobility(μ) and carrier concentration (n) of CuAlO2 films formed at different oxygen partial pressures. The films showed high electrical resistivity of 15.2 Ω cm at low oxygen partial pressure of 1 × 10−4 mbar due to the presence of mixed phase of Cu2O along with CuAlO2. The electrical resistivity of the films decreased to 3.1 Ω cm in case of the films formed at an oxygen partial pressure of 6 × 10−4 mbar due to growth of single phase of CuAlO2. Further increase in the oxygen partial pressure to 1 × 10−3 mbar the electrical resistivity of the films increased to 18.9 Ω cm due to the formation of Al2O3 phase along with CuAlO2. Higher content of Al2O3 in the films leads to dielectric behaviour and amorphous nature hence of high electrical resistivity. The low electrical resistivity of 3.1 Ω cm obtained was lower than 10.5 Ω cm reported in pulsed laser deposition [2] and 12 Ω cm obtained in dc sputtered films [23]. Wang and Gong [6] achieved low electrical resistivity of 0.14 Ω cm in Plasma Enhanced MOCVD due high carrier concentration of 4.5 × 1019 cm−3. The Hall mobility measurements indicated that the films were p-type conduction. The Hall mobility of the films increased from 0.5 to 13.1 cm2 V−1 s−1 with increase of oxygen partial pressure from 1 × 10−4 to 6 × 10−4 mbar thereafter it decreased to 0.25 cm2 V−1 s−1. The increase of Hall mobility with the oxygen partial pressure was due to the improvement in the crystallinity of the films. At higher oxygen partial pressures the decrease in the Hall mobility may be due to the presence of Al2O3 phase with high resistivity in the films. The carrier concentration of the films decreased from 8.9 × 1017 to 1.5 × 1017 cm−3 with the increase of oxygen partial pressure from 1 × 10−4 to 6 × 10−4 mbar thereafter it increased to 4.5 × 1017 cm−3 at higher oxygen partial pressure of 1 × 10−3 mbar. In the literature it was reported that the carrier concentration was in the range 1.3 × 1017–4.5 × 1019 cm−3. The large variation in the carrier concentration was due to the preparation of films using different deposition techniques and process parameters.
Optical properties
The optical transmittance and reflectance of the films were recorded in the wavelength range 350–2500 nm. Figure 5. shows the wavelength dependence of optical transmittance of CuAlO2 films formed at different oxygen partial pressures. The optical transmittance is high in the visible region. The low optical transmittance at oxygen partial pressures <6 × 10−4 mbar was due to the presence of insufficient oxygen during sputtering hence the presence of mixed phase of Cu2O and CuAlO2. As the oxygen partial pressure increased to ≥6 × 10−4 mbar the stoichiometry improved because of the decrease in the density of defect centers in the films. The light scattered by the process of reflection decreased with the decrease of defect centers, hence increase in the optical transmittance. The optical transmittance at wavelengths greater than 700 nm increased from 56% to 76% with the increase of oxygen partial pressure from 1 × 10−4 to 3 × 10−3 mbar. A sharp absorption edge was observed around the wavelength 500 nm and shifted towards lower wavelength with the increase of oxygen partial pressure. The optical absorption coefficient (α) of the films was evaluated from the optical transmittance (T) and reflectance (R) data using the relation
where t is the film thickness. The dependence of α on the photon energy (hν) was fitted to the relation for direct allowed transitions.
where A is the absorption edge width parameter and Eg the optical band gap of the film.
Extrapolation of the linear portion of the plots of (α hν)2 versus photon energy of CuAlO2 films to α = 0 resulted in the optical band gap of the films. The optical band gap of the films increased from 3.1 to 3.73 eV with the increase of oxygen partial pressure from 2 × 10−4 to 1 × 10−3 mbar. The optical band gap was not matched with the reported CuO and Cu2O values of 1.50 and 2.10 eV [24, 25] and Al2O3 value of 9.0 eV [26]. The single phase CuAlO2 films formed at an oxygen partial pressure of 6 × 10−4 mbar exhibited an optical band gap of 3.54 eV, which is good agreement with the reports of Brus [27] and Yanagi et al. [28]. However in the literature large optical band gaps of 3.75 and 3.66 eV were reported by Gong et al. [10] and Banerjee et al. [15] in metal organic chemical vapour deposited and dc sputtered films, respectively. This wider optical band gap was caused by quantum confinement because the excitation confinement in semiconductor nanocrystals leads to the development of discrete transitions to the excited electronic states with higher oscillator strength and to band gaps that increase as an inverse function of crystallite size [29, 30].
The quality of a transparent conducting oxide will be judged by the parameter of figure of merit. The figure of merit of the film was calculated from the optical transmittance and sheet resistance data using the Haack’s relation [31].
ρ is the films electrical resistivity and T the average optical transmittance in the wavelengths above 700 nm. The variation in the figure of merit of CuAlO2 with different oxygen partial pressure is shown in Fig. 6. Figure of merit of the films increased from 0.11 Ω−1 to 0.87 Ω−1 with increase of oxygen partial pressure from 1 × 10−4 mbar to 6 × 10−4 mbar afterwards it decrease to 0.17 Ω−1 with further increasing the oxygen partial pressure upto 1 × 10−3 mbar. The increase of figure of merit at lower oxygen partial pressure was due to the decrease in the electrical resistivity. The decrease at higher oxygen partial pressure was due to the increase of electrical resistivity though the films showed high optical transmittance.
Conclusions
Dc magnetron sputtering technique was employed for preparation of CuAlO2 films on glass substrate under various oxygen partial pressures in the range 5 × 10−5–3 × 10−3 mbar. The variation of cathode potential on the oxygen partial pressure was explained in terms of oxidation of sputtering target. The influence of oxygen partial pressure on the structural, electrical and optical properties of CuAlO2 films was systematically studied. X-ray diffraction studies revealed that the films were polycrystalline in nature. The films formed at an oxygen partial pressure <6 × 10−4 mbar were mixed phase of Cu2O and CuAlO2, while those deposited at an oxygen partial pressure of 6 × 10−4 mbar were single phase CuAlO2. The single phase films showed the electrical resistivity of 3.1 Ω cm. The high electrical resistivity of 18.9 Ω cm in case of the films formed at high oxygen partial pressure of 1 × 10−3 mbar was due to the presence of additional phase of Al2O3 along with CuAlO2. Hall mobility measurements indicated that the films were p-type in conduction. The single phase films showed Hall mobility of 13.1 cm2 V−1 s−1 and carrier concentration of 1.5 × 1017 cm−3. The optical transmittance of the films (at wavelength 700 nm) increased from 56 % to 76 % and optical band gap increased from 3.1 eV to 3.73 eV with the increase of oxygen partial pressure from 2 × 10−4 mbar to 1 × 10−3 mbar. In conclusion, p-type CuAlO2 films with electrical resistivity of 3.1 Ω cm, Hall mobility of 13.1 cm2 V−1 s−1, optical transmittance of 71% and optical band gap of 3.54 eV were obtained at an oxygen partial pressure of 6 × 10−4 mbar.
References
A. Kudo, H. Yanagi, K. Ueda, H. Hosono, H. Kawazoe, Y. Yano, Appl. Phys. Lett. 75, 2851 (1999)
H. Kawazoe, M. Yasukawa, H. Hyodo, M. Kurita, H. Yanagi, H. Hosono, Nature 389, 939 (1997)
H. Hiramatsu, K. Ueda, H. Ohta, M. Orita, M. Hirano, H. Hosono, Appl. Phys. Lett. 81, 598 (2002)
H. Yanagi, H. Kawazoe, A. Kudo, M. Yasukawa, H. Hosono, J. Electrochem. Soc. 4, 427 (2000)
H. Ohta, M. Orita, M. Hirano, I. Yagi, K. Ueda, H. Hosono, J. Appl. Phys. 91, 3074 (2002)
Y. Wang, H. Gong, Chem. Vapor Depos. 6, 285 (2000)
K. Ueda, S. Inoue, S. Hirose, H. Kawazoe, H. Hosono, Appl. Phys. Lett. 77, 2701 (2000)
G. Thomas, Nature 389, 907 (1997)
S. Ibuki, H. Yanagi, K. Ueda, H. Kawazoe, H. Hosono, Appl. Phys. Lett. 78, 367 (2000)
H. Gong, Y. Wang, Y. Luo, Appl. Phys. Lett. 76, 3959 (2000)
M. Ohashi, Y. Lida, H. Morikawa, J. Am. Ceram. Soc. 85, 270 (2002)
K. Tonooko, K. Shimokowa, O. Nishimura, Thin Solid Films 411, 129 (2002)
S. Gao, Y. Zhao, P. Gou, N. Chen, Y. Xie, Nanotechnology 14, 538 (2003)
N. Tsuboi, Y. Takahashi, S. Kobayashi, H. Shimizu, K. Kato, F. Kaneko, J. Phys. Chem. Solid. 64, 1671 (2003)
A.N. Banerjee, R. Maity, C.K. Ghosh, K.K. Chattopadhyay, Thin Solid Films 440, 5 (2003); 474, 261 (2005)
C.H. Ong, H. Gong, Thin Solid Films 445, 299 (2003)
E.M. Alkoy, P.J. Kelly, Vacuum 79, 221 (2005)
L.J. Van Der Pauw, Philips Res. Rep. 13, 1 (1958)
G. Mohan Rao, S. Mohan, J. Appl. Phys. 69, 6652 (1991)
W.D. Sproul, D.J. Chistie, D.C. Carter, Thin Solid Films 491, 1 (2005)
K.B. Sundaram, A. Khan, Thin Solid Films 295, 87 (1997)
B.D. Cullity, Elements of X-ray Diffraction, 2nd edn. (Addison Wesley, London, 1978)
A.N. Banerjee, C.K. Ghosh, K.K. Chattopadhyay, Solar Energy Mater. Solar Cells 89, 75 (2005)
B. Balamurugan, B.R. Mehta, Thin Solid Films 396, 90 (2001)
A. Buljan, M. Llunell, E. Ruiz, P. Alemany, Chem. Mater. 13, 338 (2001)
M. Henyk, D. Wolfframm, J. Reif, Appl. Surf. Sci. 168, 263 (2000)
L. Brus, J. Chem. Phys. 80, 4403 (1984)
H. Yanagi, S. Inoue, K. Ueda, H. Kawazoe, H. Hosono, J. Appl. Phys. 88, 4159 (2000)
J.R. Heath, Science 270, 1315 (1995)
Z.H. Lu, D.J. Lockwood, J.M. Baribeau, Nature 378, 258 (1995)
G. Haacke, J. Appl. Phys. 47, 4086 (1976)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sivasankar Reddy, A., Sreedhara Reddy, P., Uthanna, S. et al. Characterization of CuAlO2 films prepared by dc reactive magnetron sputtering. J Mater Sci: Mater Electron 17, 615–620 (2006). https://doi.org/10.1007/s10854-006-0007-2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10854-006-0007-2