Abstract
Hydrophobic silica aerogels have been prepared using the rapid supercritical extraction (RSCE) technique. The RSCE technique is a one-step methanol supercritical extraction method for producing aerogel monoliths in 3 to 8 h. Standard aerogels were prepared from a tetramethoxysilane (TMOS) recipe with a molar ratio of TMOS:MeOH:H2O:NH4OH of 1.0:12.0:4.0:7.4 × 10−3. Hydrophobic aerogels were prepared using the same recipe except the TMOS was replaced with a mixture of TMOS and one of the following organosilane co-precursors: methytrimethoxysilane (MTMS), ethyltrimethoxysilane (ETMS), or propyltrimeth-oxysilane (PTMS). Results show that, by increasing the amount of catalyst and increasing gelation time, monolithic aerogels can be prepared out of volume mixtures including up to 75% MTMS, 50% ETMS or 50% PTMS in 7.5–15 h. As the amount of co-precursor is increased the aerogels become more hydrophobic (sessile tests with water droplets yield contact angles up to 155°) and less transparent (transmission through a 12.2-mm thick sample decreases from 83 to 50% at 800 nm). The skeletal and bulk density decrease and the surface area increases (550–760 m2/g) when TMOS is substituted with increasing amounts of MTMS. The amount of co-precursor does not affect the thermal conductivity. SEM imaging shows significant differences in the nanostructure for the most hydrophobic surfaces.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Silica aerogels are porous ceramic materials consisting of 90–99% air by volume, with high surface area, low density, low thermal and electrical conductivity, and visible transparency. This unique combination of properties makes them suited to a wide range of applications from materials for thermal and acoustical insulation to chemical sensors (see for example Pierre and Pajonk [1] or Akimov [2]). Silica aerogels made using tetramethoxysilane (TMOS) or tetraethyloxysilane (TEOS) are typically hydrophilic, which makes them sensitive to moisture. To prepare silica aerogels from these precursors requires an initial reaction in which some of the Si–OCH3 (in the case of TMOS) or Si–OCH2CH3 (TEOS) groups are hydrolyzed to Si–OH. The subsequent sol–gel polymerization is due to condensation reactions, which can occur between one Si–OH and one Si–OR group or two Si–OH groups, in either case forming an Si–O–Si linkage group. In the resulting sol–gel matrix, some of the Si–OH and Si–OR groups remain unreacted. The hydroxyl groups (and, to a lesser extent, the alkoxyl groups) can undergo significant intermolecular forces with water. Because aerogels are highly porous, water vapor in the air surrounding the aerogel material can be adsorbed into the aerogel matrix and cause deterioration of the aerogel nanostructure over time [3, 4].
There are a variety of ways to make aerogels, but they all stem from a basic two-step procedure. The first step is the formation of a wet gel through a sol–gel polymerization reaction of precursor chemicals. The second step is the extraction of the sol–gel solvent, which leaves a dry, rigid nanostructure behind. This drying step can be accomplished at ambient pressure if silylation through solvent exchange is performed on the wet gel prior to drying. Otherwise it is performed using low temperature supercritical extraction after exchanging the solvent with liquid CO2 (CSCE) or by direct extraction of the alcohol solvent (usually methanol or ethanol) using high-temperature alcohol supercritical extraction (ASCE). Aerogels fabricated using high-temperature supercritical extraction are more hydrophobic than those made via CSCE [5, 6].
There are three primary techniques used to make hydrophobic silica aerogels, all of which seek to replace the hydrophilic causing hydroxyl groups using surface modification. This can be done using a vapor phase treatment of the hydrophilic aerogels during drying [7] or after the aerogel is formed [8, 9]. Or silylation can be used to change the surface chemistry of the wet gels before drying. These techniques involve multiple solvent exchanges but can use ambient pressure drying (see for example Lee et al. [10] or Rao et al. [11]).
The third method, which is the focus of this work, uses organosilanes (or fluorinated organosilanes) as a co-precursor. Schwertfeger and coworkers [12, 13] used ASCE and showed that hydrophobic aerogels could be made by organically modifying the silica gels by replacing one of the four methoxy groups on TMOS with a methyl group (methyltrimethoxysilane, or MTMS), an n-propyl group, a phenol group, or a t-butyl group. Rao and Haranath [14] and Rao and Pajonk [15] used a combination of MTMS and TMOS as aerogel precursors in an ASCE process. They found that aerogels became hydrophobic when the molar ratio of TMOS/MTMS reached 0.7 and presented maximum sessile drop contact angle measurements of 140°. More recently, Štandeker et al. [16] studied the effects of MTMS and trimethylethoxysilane (TMES) on hydrophobicity using CSCE for toxic organic compound clean-up applications and achieved contact angles of 42–144° (for MTMS/TMOS molar ratios of 0.35–5). Martin et al. [6] showed that aerogels fabricated with MTMS (with maximum contact angle of 160°) could be mechanically strong. Other investigators have had some success using fluorinated co-precursors [17–19], but we note that the fluorinated compounds used in that work are relatively expensive.
We use a rapid supercritical extraction method (RSCE, see Gauthier et al. [20], Gauthier et al. [21] Anderson et al. [22] Roth et al. [23]), a one-step process in which the precursor chemicals are mixed and then immediately placed in a metal mold for processing in a hydraulic hot press. The press is used instead of the typical autoclave to heat that mold, control the pressure and allow for supercritical extraction. The RSCE process typically takes 3–8 h from the time the chemicals are mixed to the formation of the aerogel. This is considerably faster than the CSCE and ASCE processes, which involve separate gelation and aging steps as well as solvent exchange. It is also inherently safer than the ASCE processes because the volume of solvent that is placed under high temperature and pressure is so much smaller. In RSCE the amount of solvent is simply that contained within the pores of the wet gel. In the autoclave techniques (ASCE) the entire volume of the autoclave is filled with solvent. The goals of the work presented here are to present a new, faster and simpler technique for making hydrophobic aerogels using this rapid supercritical extraction process and to demonstrate that those aerogels are as hydrophobic as those made using other procedures.
2 Methods and materials
2.1 Materials
Aerogels were prepared from a modified version of the tetramethoxysilane (TMOS, CAS 681-84-5) based recipe employed in our previous work [20, 22]. To make hydrophobic aerogels, the TMOS was replaced with varying amounts of methytrimethoxysilane (MTMS, CAS 1185-55-3), ethyltrimethoxysilane (ETMS, CAS 5314-55-6) or trimethoxypropylsilane (PTMS, CAS 1067-25-0). TMOS was purchased from Sigma–Aldrich at 99+% purity. MTMS, ETMS and PTMS were purchased from Aldrich at 98%, 97+% and 97% purity, respectively. Reagent-grade methanol, acquired from Fisher Scientific, and laboratory quality deionized water were used without further treatment. The 1.5-M ammonium hydroxide catalyst solution was prepared by dilution of concentrated ammonium hydroxide (Fisher Scientific, Reagent A.C.S. grade) with deionized water.
2.1.1 Standard aerogel recipe
The standard TMOS-based recipe utilized TMOS:MeOH:H2O:NH4OH in a 1.0:12.0:4.0:7.4 × 10−3 molar ratio. The chemicals were mixed, sonicated for 5–10 min and then poured into a mold. In all cases we prepared 20-mL batches using 4.25 mL TMOS, 13.75 mL MeOH, 1.8 mL H2O and 0.135 mL of 1.5-M NH4OH (see Table 1). The mold was then placed in the hot press for immediate processing.
2.1.2 Hydrophobic aerogel recipe
For these aerogels we replaced the 4.25 mL of TMOS with 4.25-mL mixtures of TMOS/MTMS, TMOS/ETMS or TMOS/PTMS in 25–75% volume ratios (see Table 1). We also adjusted the amount of catalyst as needed (1.5–2.5 times more for the aerogels made with less than 50% TMOS). The chemicals were mixed, sonicated for 5–10 min and poured into the mold. The mold was then placed in the hot press for immediate processing.
2.2 RSCE method
In the RSCE process [20–23], supercritical extraction is achieved using a hydraulic hot press and a custom steel two-part mold as shown in Fig. 1. The mold top employed for this work is 76.2 by 108 by 12.2 mm high with two 25.4 by 25.4 by 12.2 mm square openings. The bottom piece is the same size as the top but is solid throughout. To assemble the mold we place a 0.0254-mm-thick piece of Kapton film and a 1.59-mm-thick piece of graphite between the two mold parts which are then joined by countersunk machine screws. Before filling the 8-mL cavities (formed by the square openings), the mold is coated with a high temperature dry film lubricant material to allow for easy removal of the aerogels at the end of the process. The mold is then placed between the two platens of the hydraulic hot press and another piece of the Kapton/graphite gasket is placed on top to form a seal between the mold and the hot press platen.
The processing involves five steps, which are illustrated in Fig. 2: Gelation, Heating, Equilibration, Release and Cooling. In step 1, Gelation, the mold is heated slightly to 38 °C and sealed by setting the hot press restraining force to 94 kN for a 2-min to 7-h period. The length of this step depends on the amount of co-precursor used in the precursor mixture because the addition of MTMS, ETMS, PTMS is known to slow the hydrolysis and condensation reactions [15]. The gelation period was set to 2 min for the pure TMOS gels and from 3 to 7 h for the other gels. In step 2, Heating, the mold is heated from 38 to 288 °C over a 3-h period (for a heating rate of 1.4 °C/min). During this time we expect there will still be some condensation reactions occurring. Step 3, Equilibration, allows the system to equilibrate at 288 °C for 30 min and brings all of the solvent to the supercritical state. In step 4, Release, the restraining force is lowered to 26 kN over a 30-min period. This ‘unseals’ the mold, and releases the solvent as a supercritical fluid, leaving monolithic aerogels in the mold. The system is again allowed to equilibrate for 30 min and then in step 5, Cooling, the system is cooled over a 3-h period to 38 °C (1.4 °C/min). After completion, the hot press is opened and the mold removed. The aerogels are removed from the mold and stored in small plastic containers for later testing. The entire process takes between 7.5 and 15 h (depending on the length of the Gelation step).
2.3 Characterization
The aerogels were characterized by measuring contact angle, FTIR spectra, visible light transmission, skeletal density, bulk density, thermal conductivity and surface area. To measure contact angle, a droplet of deionized water (diameter 2–2.5 mm) was placed on a flat, level aerogel surface using a hypodermic needle and syringe. A high resolution photo was taken of the water droplet and the height (h) and contact width (b) of the droplet were estimated using image-processing software. The contact angle was then estimated following the technique of Rao et al. [24] as equal to 2 × tan−1(2 h/b). Three to five different droplet images were acquired for each aerogel and average contact angles were calculated. The variation in contact angle was generally less than 2° (with the exception of the measurements for the 100% TMOS aerogel, as discussed below). A Micromeritics AccuPyc 1330 gas pycnometer was used to measure the skeletal density of the hydrophobic aerogels. Each sample was purged 99 times at the start of each measurement and the skeletal density measurements were repeated up to 5 times. Bulk density was estimated using mass and volume measurements. The uncertainty in the skeletal density was estimated using the standard deviation in the pycnometer measurements and the uncertainty in the mass measurement and was found to be about 3%. The uncertainty in the bulk density was estimated to be 7% (based on volume and mass measurement uncertainties). A Micromeritics ASAP 2010 system was used to measure the surface area of aerogels using the BET method. Aerogel samples were crushed and then degassed for 2 h at 90 °C and 4–10 h at 200 °C before testing. Uncertainties in BET surface areas were calculated to include standard deviation associated with the BET fit and the uncertainty in the mass measurement, and ranged from 2 to 4%. A Hot Disk Thermal Constants Analyzer was used to measure the thermal conductivity of the aerogel monoliths (±10%). This system uses a transient contact method to estimate thermal conductivity. A sensor is sandwiched between two similar aerogel samples and set to output 20–50 mW over a 20- to 40-s period. Thermal conductivity is then estimated using the temperature–time history. FTIR spectra were taken on a Nicolet Avatar 330 FTIR equipped with a SMART ORBIT diamond ATR attachment. A resolution of 2 cm−1 was employed and 64 scans were averaged. A UV/VIS/NIR Lambda 900 Spectrometer was used to measure the percent transmittance through 12.2-mm thick aerogel samples over a range of wavelengths from 800 to 340 nm. Spectra were taken at 4–5 different locations and then averaged. Four of the aerogels were imaged using a Zeiss EVO-50XVP scanning electron microscope. Samples were attached to round metal stages using 12-mm carbon adhesive tabs and sputter coated with a mixture of gold and palladium for 15–30 s in a Denton Desk IV sputter coater at the 20% sputter set-point to reduce charging. The aerogels were imaged at a working distance of 4–6 mm. A relatively low beam voltage of 5 kV was used with probe currents of 20 pA, 50 pA, or 120 pA in order to reduce charging.
3 Results and discussion
Table 2 summarizes the results for the different aerogels. The sample name is given in the form “TXXXY-G-C” where XXX refers to the percent volume content of TMOS in the precursor mixture, Y is used to identify the co-precursor (M = MTMS, P = PTMS, E = ETMS), G indicates the length of the Gelation processing step (hr) and C indicates the amount of catalyst used (1.5 = 1.5x, 3 = 3x, etc.).
Figure 3 shows typical standard and hydrophobic aerogel samples. As we increased the amount of co-precursor the aerogels became less transparent, more fragile and somewhat more elastic (spongier). The presence of the organosilane inhibits the condensation and hydrolysis reactions, which can result in increased gelation times. Rao and Pajonk [15] report an increase from 4 to 15 h as the MTMS/TMOS molar ratio increased from 0 to 1. At a molar ratio of 1 (50% MTMS by volume) we were able to fabricate aerogels using a 2-min gelation step (T50M-0-1). The increased temperature during the heating step of our process helps to promote gelation. However, at an MTMS/TMOS molar ratio of 3 (T25M-5-1) we were unable to make monolithic aerogels using RSCE unless we increased the gelation time to at least 5 h. Processing with ETMS and PTMS at molar ratios (to TMOS) of 0.9-1 required gelation steps of 5–7 h and required higher levels of catalyst.
Figure 4 plots contact angle measurement of sessile drops on the MTMS aerogels ranging from 25 to 100% TMOS (75–0% MTMS) for the aerogels made with a 5-h gelation step. Individual data points (solid diamonds) are plotted to give an idea of the variation in the measurement of contact angle. The horizontal line marker represents the average of the measurements. The pictures located below the x axis are representative photos of the water drops on the aerogel surfaces. As the amount of MTMS increases and the amount of TMOS decreases, the sessile contact angle increases, indicating an increase in hydrophobicity. The scatter in the measurement also decreases as hydrophobicity increases. There is a large amount of scatter in the data for the 100% TMOS aerogel and an examination of the droplet photos shows that some areas of the particular sample had higher hydrophobicity than other areas indicating significant variation on the TMOS aerogel surface. Contact angles for the ETMS and PTMS aerogels are similar to those of the MTMS aerogels (see values in Table 2). As the amount of the organically modified precursor used in the sol–gel recipe increases, the contact angle increases. However, we see no significant difference in the hydrophobicity achieved for the different ormosils at the same volume ratios.
Contact angle versus percent content (by volume) of MTMS co-precursor. The data shows significant scatter in the contact angle measurement for the 100% TMOS aerogel. The pictures are representative photos of the water drops on the aerogel surface at each volume ratio. The horizontal line indicates the average of the contact angle measurements for each case
Figure 5 compares the present contact angle data to that of Rao and Pajonk [15], Štandeker et al. [16], and Martin et al. [6] as a function of the MTMS/TMOS molar ratio. Rao and Martin used high temperature supercritical extraction (ASCE) whereas Štandeker used a CO2 supercritical extraction method (CSCE). All data shows increasing hydrophobicity (through increasing contact angle) with increasing molar ratio. The Štandeker data is significantly lower than the others, which is probably due to the use of low-temperature extraction instead of high-temperature extraction. At the lower MTMS/TMOS molar ratios the RSCE fabricated aerogels are significantly more hydrophobic than those produced using CSCE and ASCE; our results are similar at higher molar ratios.
A comparison of contact angle versus molar ratio of MTMS to TMOS. All data sets show an increase in contact angle with increasing molar ratio. The present results show higher contact angles. Anderson et al. used high temperature RSCE processing, Martin [6] and Rao [15] used high temperature ASCE processing and Štandeker [16] used low temperature CSCE processing
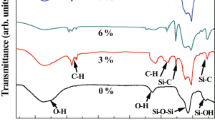
The FTIR spectra, shown in Fig. 6, demonstrate the differences in the structures of the silica aerogels that result from the inclusion of the TMOS derivatives. Peaks observed are consistent with the sol–gel literature [25]. The prominent feature in the spectrum of the 100%-TMOS-based aerogel is a broad peak due to Si–O–Si stretching (1100 cm−1). With the inclusion of MTMS, peaks due to (Si)CH3 stretching (2974 cm−1) and (Si)CH3 rocking (778 cm−1) modes are observed. Corresponding peaks are seen in the spectra of ETMS- and PTMS-based aerogels, due to ethyl and propyl groups in the aerogel matrix. As the percent of ormosil in the precursor increases, these peaks become more intense. Interestingly, peaks due to unreacted Si–OH or Si–OCH3 groups are not observed and there is no evidence of adsorbed water in these aerogel samples.
FTIR spectra of aerogels. With the inclusion of MTMS, peaks due to (Si)CH3 stretching (2974 cm−1) and (Si)CH3 rocking (778 cm−1) modes are observed. Corresponding peaks are seen in the spectra of ETMS- and PTMS-based aerogels, due to ethyl and propyl groups in the aerogel matrix. As the percentage of organosilane in the precursor increases, these peaks become more intense. Peaks due to unreacted Si–OH or Si–OCH3 groups are not observed. There is no evidence of adsorbed water in these aerogel samples (Inset is a blow up of the data from 2700 to 3100 cm−1)
An important application area for aerogels is in transparent insulating materials for lighting applications where high levels of light transmission and low levels of thermal conductivity as well as hydrophobicity are desired. Thermal conductivity results are presented in Table 2. The thermal conductivity remains fairly independent of the level and type of ormosil with values averaging about 0.035 (±10%) W/mK. However, the inclusion of the ormosils adversely affects the light transmission levels. Transmission values at 800 nm are listed in Table 2 and sample spectra are plotted in Fig. 7 (these results are for 12.2-mm thick samples). As Figs. 3 and 7 show, the 100% TMOS aerogels are the most transparent; as the percent of ormosil increases the aerogels become less transparent.
Skeletal and bulk density results are plotted in Fig. 8 and tabulated in Table 2. Both the skeletal and bulk density of the aerogel decrease as the amount of ormosil increases. The skeletal density ranges from 1.41 g/mL for T25M to 1.83 g/mL for T100 whereas the bulk density ranges from 0.048 to 0.069 g/mL for the same aerogels. These densities yield porosities in the 95–97% range. An ‘ideal’ T100 silica aerogel would be composed entirely of SiO2 (skeletal density ca. 2 g/mL) because each TMOS molecule would have undergone four condensation reactions to form four Si–O–Si bonds; however, real TMOS aerogels will have “dead ends” in their polymer chains due to unreacted Si–OH and Si–OCH3 groups, which leads to a lower skeletal density. Aerogels prepared using MTMS, ETMS, and PTMS moieties each contain more “dead ends” within the polymer matrix because the organically modified branches do not undergo condensation reactions. Moreover, as the organic branch length increases, steric hindrance will limit the direction of polymer chain growth, leading to a lower-density matrix. The aerogel made from 75% MTMS (T25M) has the lowest bulk density (0.05 g/mL) although among the 50% TMOS aerogels the PTMS aerogels have the lowest skeletal and bulk densities.
The surface area of the standard TMOS aerogel is significantly lower than that of the hydrophobic aerogels although, as Rao and Haranath [14] report, the differences in the hydrolysis and condensation processes when MTMS is present leads to different microstructures so direct comparison is problematic. We measure a maximum surface area (770 m2/g) for the T50M aerogel and then the value decreases as more MTMS is added to the precursor mixture. We find an increase to a maximum surface area and then a decrease with increasing amount of MTMS. This is in agreement with the results of Martin et al. [6] and Štandeker et al. [16]. In contrast, Rao and Pajonk [15] show decreasing surface area with increasing MTMS; however, their surface areas (~1000 m2/g) are significantly higher than the ones presented here.
Four aerogels (T100, T25M, T50M and T75M) were imaged using SEM at several different magnifications to compare the structure of the aerogels at various scales. The results at 5 and 50 kX are shown in Fig. 9. The T100 aerogel shows a cloudlike, airy nanostructure with visible pores smaller than 100 nm. The T25M and T50M aerogels are similar. The porous silica lattice is clearly visible, with portions of the aerogels containing denser rounded clusters of silica. The 50% MTMS (T50M) aerogel appears to contain fewer large pores than the 25% MTMS aerogel (but we note that this may simply be the result of the sections of each aerogel viewed). T25M has the coarsest and most irregular structure of all. It shows a convoluted silica lattice with branchlike “teeth” of porous silica. The structure appears more amorphous, with large (greater than 100 nm) pores scattered throughout a silica network. This variation in nanostructure can be related to the hydrophobicity. As more MTMS is added, the aerogel structure appears to become rougher and more irregular. The T100 aerogel was relatively smooth and it was necessary to magnify it to 40 kX to see the pores within the nanostructure. The surfaces of the 25 and 50% MTMS aerogels were more uneven and the pores were visible at 15 kX. The 75% MTMS aerogel was highly irregular with jagged extensions of silica. Some pores were visible at 1 kX, and the porous silica lattice could be seen clearly at 5 kX. These observations suggest that the aerogels with more MTMS have a higher roughness ratio and lower surface fraction and would be more hydrophobic. This is in agreement with the contact angle results, with contact angles as high as 155° for 75% MTMS aerogels, and suggests that the surface structure as well as the surface chemistry contribute to the hydrophobic nature of the aerogel.
RSCE silica aerogels fabricated from TMOS alone (with no added ormosil) lose hydrophobicity over time due to the hydrophilic surface chemistry. We observed that the RSCE TMOS aerogels exposed to the ambient environment for more than a week or two would become noticeably hydrophilic. However, 2 years after fabrication, our organically modified aerogels (T25M, T50M and T75M) still maintained high levels of hydrophobicity, with measured contact angles in excess of 140°.
4 Conclusion
In summary, we have demonstrated that we can fabricate hydrophobic aerogels using the rapid supercritical extraction method and have characterized the hydrophobic aerogels using a battery of analytical techniques. The RSCE technique is a one-step precursor-to-aerogel method that does not require separate gelation or solvent exchange steps. Our aerogels are as hydrophobic (or more so) than those fabricated using conventional supercritical extraction with CO2 or high temperature solvent extraction. We were able to fabricate these hydrophobic aerogels in 7–15 h which is much faster than methods reported in the literature, which require 4–5 days (for gelation, solvent exchange and extraction). The low densities and high surface areas of the hydrophobic aerogels produced via the RSCE method render them attractive for a wide variety of applications.
References
Pierre AC, Pajonk GM (2002) Chemistry of aerogels and their applications. Chem Rev 102:4243–4265
Akimov YK (2003) Fields of application of aerogels (review). Instrum Exper Tech 46(3):287–299
Miner M, Hosticka B, Norris P (2004) The effects of ambient humidity on the mechanical properties and surface chemistry of hygroscopic silica aerogel. J Non-Cryst Solids 350:285–289
Rao AP, Rao AV, Bangi UKH (2008) Low thermal conductive, transparent and hydrophobic ambient pressure dried silica aerogels with various preparation conditions using sodium silicate solutions. J Sol–Gel Sci Technol 47(1):85–94
Hüsing N, Schwertfeger F, Tappert W, Schubert U (1995) Influence of supercritical drying fluid on structure and properties of organically modified silica aerogels. J Non-Cryst Solids 186:37–43
Martín L, Ossó JO, Ricart S, Roig A, García O, Sastre R (2008) Organo-modified silica aerogels and implications for material hydrophobicity and mechanical properties. J Mater Chem 18(2):207–213
Yokogawa H, Yokoyama M (1995) Hydrophobic silica aerogel. J Non-Cryst Solids 186:23–29
Lee KH, Kim SY, Yoo KP (1995) Low-density, hydrophobic aerogels. J Non-Cryst Solids 186:18–22
Smirnova I, Suttiruengwong S, Arlt W (2004) Feasibility study of hydrophilic and hydrophobic silica aerogels as drug delivery systems. J Non-Cryst Solids 350:54–60
Lee C, Kim G, Hyun S (2002) Synthesis of silica aerogels from waterglass via new modified ambient drying. J Mater Sci 37(11):2237–2241
Rao AP, Rao AV, Pajonk GM (2005) Hydrophobic and physical properties of the two step processed ambient pressure dried silica aerogels with various exchanging solvents. J Sol–Gel Sci Technol 36(3):285–292
Schwertfeger F, Glaubitt W, Schubert U (1992) Hydrophobic aerogels from Si(OMe)4/MeSi(OMe)3 mixtures. J Non-Cryst Solids 145:85–89
Schwertfeger F, Hüsing N, Schubert U (1994) Influence of the nature of organic groups on the properties of organically modified silica aerogels. J Sol–Gel Sci Technol 2(1):103–108
Rao AV, Haranath D (1999) Effect of methyltrimethoxysilane as a synthesis component on the hydrophobicity and some physical properties of silica aerogels. Microporous Mesoporous Mater 30(2–3):267–273
Rao AV, Pajonk GM (2001) Effect of methyltrimethoxysilane as a co-precursor on the optical properties of silica aerogels. J Non-Cryst Solids 285(1–3):202–209
Štandeker S, Novak Z, Knez Ž (2007) Adsorption of toxic organic compounds from water with hydrophobic silica aerogels. J Colloid Interface Sci 310(2):362–368
Reynolds JG, Coronado PR, Hrubesh LW (2001) Hydrophobic aerogels for oil-spill clean up––synthesis and characterization. J Non-Cryst Solids 292(1–3):127–137
Hrubesh L, Coronado P, Satcher J (2001) Solvent removal from water with hydrophobic aerogels. J Non-Cryst Solids 285(1–3):328–332
Roig A, Molins E, Rodríguez E, Martínez S, Moreno-Mañas M, Vallribera A (2004) Superhydrophobic silica aerogels by fluorination at the gel stage. Chem Comm 2004(20):2316–2317
Gauthier BM, Bakrania SD, Anderson AM, Carroll MK (2004) A fast supercritical extraction technique for aerogel fabrication. J Non-Cryst Solids 350:238–243
Gauthier BM, Anderson AM, Bakrania SD, Mahony MK, Bucinell RB (2008) Method and device for fabricating aerogels and aerogel monoliths obtained thereby. U.S. Patent 7,384,988. 10 June 2008
Anderson AM, Wattley CW, Carroll MK (2009) Silica aerogels prepared via rapid supercritical extraction: effect of process variables on aerogel properties. J Non-Cryst Solids 355(2):101–108
Roth TB, Anderson AM, Carroll MK (2008) Analysis of a rapid supercritical extraction aerogel fabrication process: prediction of thermodynamic conditions during processing. J Non-Cryst Solids 354(31):3685–3693
Rao AV, Kulkarni M, Pajonk G, Amalnerkar D, Seth T (2003) Synthesis and characterization of hydrophobic silica aerogels using trimethylethoxysilane as a co-precursor. J Sol–Gel Sci Technol 27(2):103–109
Fidalgo A, Ciriminna R, Ilharco LM, Pagliaro M (2005) Role of the alkyl-alkoxide precursor on the structure and catalytic properties of hybrid sol–gel catalysts. Chem Mater 17:6686–6694
Acknowledgements
The Union College Aerogel Fabrication, Characterization, and Applications Laboratory has been funded by grants from the National Science Foundation (NSF MRI CTS-0216153, NSF RUI CHE-0514527, NSF MRI CMMI-0722842, and NSF RUI CHE-0847901), the American Chemical Society’s Petroleum Research Fund (ACS PRF 39796-B10), the Union College Faculty Research Fund and Union College Student Internal Education Fund. ECG received a Davenport Summer Research Fellowship from Union College. The SEM instrument was funded through grants from the National Science Foundation (NSF MRI 0619578) and New York State Assembly RESTORE-NY. The authors are grateful to Professor Brad Bruno for the use of the camera employed for contact angle measurements and helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anderson, A.M., Carroll, M.K., Green, E.C. et al. Hydrophobic silica aerogels prepared via rapid supercritical extraction. J Sol-Gel Sci Technol 53, 199–207 (2010). https://doi.org/10.1007/s10971-009-2078-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-009-2078-z