Abstract
A facile and versatile method based on hydrolysis and subsequent condensation of silica alkoxides through sol–gel approach is proposed to obtain an internal-hydrophobic and surface-hydrophilic silica aerogel monoliths. Wet gel monoliths were modified in turn by the hydrophobic agent trimethychlorosilane (TMCS) and hydrophilic modifier 3-aminopropylsilanetriol and finished with supercritical CO2 drying. The silica aerogels were characterized by scanning electron microscopy (SEM), fourier transform infrared spectroscopy (FT-IR), water contact angle, and microcomputer-controlled electron universal tests. The characterization on microstructure of prepared internal-hydrophobic and surface-hydrophilic silica aerogel monoliths indicated that the monoliths have a typical network structure with a porous interior, which exhibits hydrophobic performance (Max. contact angle = 137°) and a hydrophilic surface (Min. contact angle = 60°). The dually modified silica aerogel monoliths were coated with a pure acrylic emulsion layer through which the compressive strength was enhanced greatly which proved that the proposed approach is valuable to silica aerogel modification.

Highlights
-
The monoliths have a typical network structure with a porous interior, which exhibits hydrophobic performance (Max. contact angle = 137°), and a hydrophilic surface (Min. contact angle = 60°).
-
The dually modified silica aerogel monoliths were coated with a pure acrylic emulsion layer through which the compressive strength was enhanced greatly.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Silica aerogel is a widely used nanoporous and amorphous solid materials with distinct advantages such as high specific surface area (500–1200 m2 g−1), high porosity (80–99.8 %), ultra-low dielectric constant (k = 1.0–2.0), low index of refraction (~1.05), etc. [1,2,3,4,5], which has attracted a lot of attention in several fields: transparent thermal insulators [6,7,8], Cherenkov detectors [9], monolithic precursors to glasses [10], and catalysts [11]. Especially the remarkable structure of silica aerogels has found applications in high-temperature-resistant and spacecraft thermal insulations [12,13,14].
Silica aerogels can be simply classified into hydrophilic and hydrophobic aerogels according to their properties. Venkateswara Rao [15] utilized various alkyl-alkoxy/chloro silane compounds to modify the silica aerogels surface; among these compound modifiers, it was found that tetramethylsilane can contribute to hydrophilic performance. Sarawade [16] discovered that the modifier trimethychlorosilane (TMCS) could completely change hydrophobic aerogels of beads’ surface into hydrophilic after 1-h thermal treatment >400 °C, and they have obtained hydrophilic silica aerogel monoliths with high surface area, good pore distribution, and low density.
On the contrary, silica aerogel monoliths without modification were hydrophilic due to the existence of moisture absorption group—hydrophilic silanol group—in the whole aerogel monoliths, inducing silica aerogel that absorbs water easily. This performance would damage the internal structure of silica aerogel and hinders its applications in many fields. Therefore, researches related to aerogel surface hydrophobic modification were reported. For example, Parvathy Rao [17] had successfully prepared hydrophobic silica aerogels through ambient pressure drying method using various silylating agents. Also Bhagat [18] had reported a novel precursor (sodium silicate) to synthesize super-hydrophobic silica aerogel powders by the same method. The currently obtained maximum contact angle from literature was up to 150°. Although many organic aerogels can get super-hydrophobic properties for special surface microstructure by modified special group [19], their medium temperature and fireproof property are still issues. Silica aerogel has advantages than the organic aerogels in medium temperature range, and the internal-hydrophobic and surface-hydrophilic silica aerogels can find applications in waterborne coatings due to their outstanding heat-insulating property and excellent flame-retardant property.
It is well known that the mechanical property of hydrophilic and hydrophobic silica aerogels was not good enough to satisfy practical requirements, and a large number of studies aimed to reduce its water absorption and to enhance the compressive strength. For instance, fiber [20, 21] or a macromolecule layer was introduced or coated on the surface during the process of aerogel preparation to enhance the mechanical compressive strength. According to “A dissolves A” rule, the internal porous structure of a complete hydrophilic aerogel monoliths with hydrophilic coatings can be damaged by water absorption; and the internal-hydrophilic and surface-hydrophobic aerogel monoliths with hydrophilic coatings are hard to bond with each other, as well as a complete hydrophobic aerogel monoliths with hydrophilic coatings. However, the internal-hydrophobic and surface-hydrophilic aerogel monoliths with hydrophilic coatings should be an ideal material not only with an integral and hard-to-damage internal structure but also has the probability to perfectly bond with each other.
A novel approach to obtain such material is proposed in this study. The paper is devoted to investigate the surface chemistry modification (Fig. 1) and the approach to enhance the compressive strength of silica aerogel monoliths. Silica aerogel monoliths were prepared by sol–gel approach using tetraethyl silicate as source, ethanol as solvent, and trimethychlorosilane and 3-aminopropyl-3-hydroxylsilanes as hydrophobic and hydrophilic modification agents, respectively. Subsequently, the internal-hydrophobic and surface-hydrophilic silica aerogel with different hydrophilic gradient monoliths was prepared through super critical CO2 drying; finally strong silica aerogel monoliths were synthesized after pure acrylic emulsion coating.
2 Experiments
2.1 Materials
Reagent-grade tetraethyl silicate (TEOS), dehydrated alcohol (EtOH), nitric acid (HNO3), ammonia (NH3·H2O, 25–28 Wt %), n-hexane, TMCS, and 3-aminopropylsilanetriol (3-APST) were used as raw chemicals (from Sinopharm Co., Ltd and Onichem Specialities Co., Ltd, China).
2.2 Methods
2.2.1 Synthesis of silica gel monoliths
Silica sols were prepared by mixing TEOS, EtOH, H2O, and HNO3 at a molar ratio of 1:4:7:1.45 × 10−2, respectively, in solution with a pH (pH = 4), followed with a water-bath treatment at 60 °C (about 2 h) to accelerate the hydrolysis of TEOS. Silica alcogels can be obtained by casting 5 ml of prepared silica sol into the mould, followed by addition of aqueous ammonia solution (4 × 10−4 mol). After sealing of the prepared alcogels for 3 days at room temperature, silica gel monoliths can be obtained.
2.2.2 Hydrophobic modification of silica wet gel monoliths
Silica wet gels were modified by n-hexane/TMCS (hydrophobic reagent) solution (20:1 in volume) for 3 times, each modification lasted for 48 h; the modified gels were kept washed with n-hexane to remove residual chemicals until no precipitation emerged when AgNO3 was dropped into the residual liquid, indicating that no more Cl− exist, and finally pure hydrophobic silica gel monoliths can be produced.
2.2.3 Synthesis of the internal-hydrophobic and surface-hydrophilic silica aerogel monoliths
Washing the hydrophobic gel monoliths with anhydrous ethanol for 24 h and exchanging the n-hexane in the sol every 1 h, 16 hydrophobic gel monoliths coded from 0 to 15 were modified with 3-APST/EtOH two times (each time for 24 h). The amount of EtOH was 10 ml for each sample with a corresponding dosage of 3-aminopropylsilanetriol varied from 0 to 1.5 ml at a rate of 0.1 ml. After hydrophilic treatment, every 3 h anhydrous ethanol was used to wash the gel to remove the residual hydrophilic agent. Finally, the internal-hydrophobic and surface-hydrophilic silica aerogel monoliths can be obtained after the dually modified wet gel monoliths were subjected to supercritical CO2 drying.
The entire synthetic pathway of the internal-hydrophobic and surface-hydrophilic silica aerogel monoliths is demonstrated in Fig. 2.
2.2.4 Synthesis of the strong silica aerogel monoliths
Pure acrylic emulsion (thickness ≈ 1 mm) as a kind of the waterborne paints includes many hydrophilic groups with advantages of ease of handling, mixing with water during application, and drying very quickly. This silica aerogel monoliths was painted two times by pure acrylic emulsion using a brush, with intervals of 30 min. The coated silica aerogel monoliths was subjected to drying at room temperature and ambient pressure for 2 h. And the thickness of the coating is about 1.0 mm. So hydrophilic groups of this paint and the surface of the modified silica aerogel monolith make good bonding properties of interface of this coating and the monolith to enhance their compressive strength.
2.3 Characterization
The dually modified silica gel monoliths were dried for 6 h (at 8.0 MPa, 80 °C) through supercritical CO2 drying device (HA22-5006). FT-IR spectra of thin slice samples were recorded from 400 cm−1 to 4000 cm−1 using IRPESTIGE-21 after mixing with fully dried KBr at the ratio of 1:(15–20). Water contact angle was measured using distilled water by electron universal tester (CMT-6104), and the weight of each water droplet is 5 mg. The images of water contact angle were taken by a separated camera (Canon D70) and used photoshop drawing tool to calculate the angle. The compressive strength of the silica aerogel monoliths were detected by microcomputer control electron universal testing machines (CMT-6104), and the monoliths were placed in the center with a compression speed of 5 mm min−1. The morphology of silica aerogel monolith was examined using scanning electron microscope (SEM JSM-6510).
3 Results and discussion
3.1 Scanning electron microscopy
Figure 3 shows the morphology and structure of the modified silica aerogel monoliths by SEM.
It can be seen from Fig. 3 that a porous network structure was achieved after modifications for the internal portion. This is a typical porous morphology of silica aerogels, which is no different from the pore morphology of the unmodified silica aerogels.
3.2 FT-IR spectra
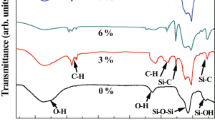
Figure 4 shows FT-IR spectra of samples (coded as a, b, c, and d) with different hydrophilic agent dosages. In order to observe the modification effects of the monoliths, samples with the best hydrophilicity (1.0 ml) were scraped into three slight layers from outer surface to the internal of monoliths under the same condition. Figure 5 shows the infrared spectra of different parts of the same sample. It is found that there is no peak near 3450 cm−1 for sample d with hydrophilic agents of 0 ml both in Figs. 4 and 5 (internal layer), which means that -OH did not exist [9], indicating a successful hydrophobic modification. However, samples a–c have a wide peak near 3450 cm−1 both in Figs. 4 and 5 (middle layer and outer layer), and these phenomena represent that the anti-symmetric -OH stretching vibration exists, suggesting that hydrophilic modification -OH was grafted on the sample surface. Also from Fig. 4, it can be seen that peak located near 3450 cm−1 was enhanced with the increase of the hydrophilic agents. On the other hand, peak at the same wavenumber in Fig. 5 was enhanced from inner layer to outer layer after hydrophilic modification, indicating that dual modification ensured the internal-hydrophobic and surface-hydrophilic property.
Peaks near 457 cm−1 and 1041 cm−1 represent Si-O-Si bending vibration and anti-symmetric stretching vibration [16, 22]; and peak near 1558 cm−1 represents the existence of N-H bonds in sample, indicating that hydrophilic agent 3-aminopropyl-3-hydroxysilane grafted onto the surface of silica aerogel monoliths which ensures a hydrophilic modification of external silica aerogels. Based on these results, the internal-hydrophobic and surface-hydrophilic performance was successfully obtained on silica aerogel monoliths. Peak located near 2980 cm−1 is due to the C-H stretch in Si-CH3 [16], which demonstrated that the modified silica aerogels skeleton had grafted the Si-CH3 bonds and replaced most of -OH bonds.
3.3 Water contact angle test
Figure 6 displays the water contact angles of different samples. It can be seen that sample 0 without any hydrophilic agent exhibits the largest contact angle of 137°; sample 1 with minimum hydrophilic agent dosage has a contact angle of 112°; sample 2 has the smallest contact angle and best hydrophilic performance which proved that 1.0 ml is the optimized dosage. In addition, the contact angle decreased with the increase of the hydrophilic agent amount, with gradually increased hydrophilicity, and there were obvious changes in water contact angle until hydrophilic dose reached a limit.
In order to give readers a more intuitive description on hydrophilic modification effects, water contact angles at different parts of the same sample 2 were tested and compared as shown in Fig. 7.
From Fig. 7, the minimum contact angle (60°) is of outer layer, the middle layer has a contact angle of 95°, while the inner layer exhibits the biggest angle of 116°. It can be deduced that hydrophilicity decreased gradiently from the outer to the inner, while keeping an internal-hydrophobic and surface-hydrophilic property as expected.
3.4 Characterization of hydrophilic degree
From Fig. 8, it is obvious that the sample with 0 ml hydrophilic agents is completely floating on water, which mean that it is highly hydrophobic; sample with 0.3 ml is submerged in water; sample with 0.7 ml of hydrophilic agents is submerged in water deeper; while sample with 1.0 ml and 1.5 ml of hydrophilic agents are almost totally submerged in water. The more the hydrophilic agent used, and the deeper the sample is submerged in water, the higher hydrophilic property theagents exhibit. Note that this case could reach a saturation point that needs further study. Change of hydroxyl group content is considered to be a key factor in water absorption. Analysis methods as described in literature [23] were used to determine the content of hydroxyl group. The specific method steps are as follows: 0.1 g outlayer samples of SiO2 aerogel monoliths, 1.25 ml ethanol, and 3 ml NaCl (20 wt%) aqueous solution were mixed first. The mixture was then titrated with HCl (0.1 mol l−1) to pH value of 4, and then NaOH (0.1 mol l−1) was added slowly until the pH value of the mixture reached 9.0 and allowed to remain in this situation for 20 s. Then the hydroxyl number per square nanometer silica aerogel monoliths outlayer surface was calculated by Eq. 1: [23]
where C represents the concentration of NaOH solution, mol l−1; V represents the volume of NaOH (0.1 mol l−1) solution consumed when the pH value of the mixture was changed from 4.0 to 9.0, mL; NA is Avogadro constant; m the weight of outlayer silica aerogel monoliths samples, g; and S the specific surface area, nm2 g−1.
Table 1 shows that there were fewer hydroxyl groups in TMCS-modified silica aerogel monoliths, but there were more hydroxyl group modified by 3-APST. With the increasing of 3-APST concentration, the number of hydroxyl group per unit area in silica aerogel monoliths increased. Hydrophilic degree of samples also increased with the number of hydroxyl group per unit area. This result is identical with Fig. 8.
3.5 BET measurements
Surface area was measured based on the amount of N2 gas adsorbed at various partial pressures, and a single condensation point (p/po = 0.99) was used to determine the pore size and cumulative pore volume. Before N2 adsorption, the sample was degassed at 200 °C. Pore size distributions were calculated from the desorption isotherms. The specific surface area for the silica aerogel monoliths was 1031.9 m2 g−1.
The physisorption isotherms obtained for the silica aerogel monoliths are Type IV, which is the characteristic feature of the mesoporous materials [14]. The desorption cycles of the isotherms showed a hysteresis loop for all the samples that is generally attributed to the capillary condensation occurring in the mesopores as shown in Fig. 9.
3.6 The comparison of the compressive strength
The silica aerogel is crushed under constant pressure. Figure 10 shows the compressive strength of the modified monoliths before and after coated with pure acrylic emulsion. It is clear that the compressive strength of sample with the amount of hydrophilic agents used increased from 0.10 to 0.66 MPa, the sample without modification (0 ml) shows the poorest compressive strength after coating due to its hydrophobic property, while the pure acrylic emulsion is hydrophilic, and according to the rule of “A dissolves A” they could not bond with each other. Sample with 1.0 ml exhibited the highest compressive strength proving a hydrophilic structure. The compressive strength of the monoliths was enhanced to a maximum value of 3.44 MPa by the increase of amount of hydrophilic agents after coating with pure acrylic emulsion. Through the comparasion of two samples in Fig. 10, it can be concluded that coating with pure acrylic emulsion reinforced the compressive strength greatly.
4 Conclusions
The internal-hydrophobic and surface-hydrophilic silica aerogel monoliths were successfully obtained through a novel dual modification method. The prepared monoliths have a typical network shape with porous internal structure with the specific surface area of 1031.9 m2 g−1. After dual modification, the hydrophilicity of the monolith increased and the degree varied at different parts of the same sample depending on hydrophilic agents and amounts. After coating with pure acrylic emulsions, the compressive strength of the monoliths was improved greatly which proved that the dual modification method is an efficient and successful aerogel modification method. The compressive strength of the silica aerogel monoliths coated with pure acrylic emulsion is increased by >5 times than that of uncoated due to the hydrophobic and hydrophilic nature.
References
Tamon H, Sone T, Okazaki M (1997) Control of mesoporous structure of silica aerogel prepared from TMOS. J Colloid Interf Sci 188(1):162–167
Leventis N, Elder IA (1999) Durable modification of silica aerogel monoliths with fluorescent 2,7-diazapyrenium moieties. Sensing oxygen near the speed of open-air diffusion. Chem Mater 11(10):2837–2845
Mohanan J, Brock SH (2003) Influence of synthetic and processing parameters on the surface area, speciation, and particle formation in copper oxide/silica aerogel composites. Chem Mater 15(13):2567–2576
Prakash S, Jeffreybrinker C, Hurd A (1995) Silica aerogel films at ambient pressure. J Non-Cryst Solids 190(3):264–275
Soleimanidorcheh A, Abbasi MH (2008) Silica aerogel; synthesis, properties and characterization. J Mater Process Technol 199(1–3):10–26
Alnaief M, Smirnova I (2011) In situ production of spherical aerogel microparticles. J Supercrit Fluids 55(3):1118–1123
Pierre A, Pajonk GRM (2002) Chemistry of aerogels and their applications. Chem Rev 102(11):4243–4265
Zhang HX, Qiao YJ, Zhang XH (2010) Structural and thermal study of highly porous nanocomposite SiO2-based aerogels. J Non-Cryst Solids 356(18–19):879–883
Wang SW, Kim TY, Yun SH (2010) Effect of surface modification conditions on the synthesis of mesoporous crack-free silica aerogel monoliths from waterglass via ambient-drying. Microporous Mesoporous Mater 130(1–3):295–302
Tamon H, Kitamura T, Okazaki M (1998) Preparation of silica aerogel from TEOS. J Colloid Interf Sci 197(2):353–359
Kim S, Shin S, Lenhardt J (2013) Deterministic control over high-Z doping of polydicyclopentadiene-based aerogel coatings. ACS Appl Mater Interfaces 5(16):8111–8119
Jeong AY, Mankoo S, Pyokim D (2000) Characterization of hydrophobic SiO2 powders prepared by surface modification on wet gel. J Sol-Gel Sci Technol 19(1–3):483–487
Schmidt M, Schwertfeger F (1998) Applications for silica aerogel products. J Non-Cryst Solids 225(1):364–368
Shi JJ, Lu LB, Guo WT (2013) Heat insulation performance, mechanics and hydrophobic modification of cellulose–SiO2 composite aerogels. Carbohyd Polym 98(1):282–289
Venkateswara Rao A, Kulkarni M, Amalnerkar DP (2003) Surface chemical modification of silica aerogel using various alkyl-alkoxy/chloro silanes. Appl Surf Sci 206(1–4):262–270
Sarawade P, Kim JK (2011) Synthesis of sodium silicate-based hydrophilic silica aerogel beads with superior properties: effect of heat-treatment. J Non-Cryst Solids 357(10):2156–2162
Parvathy Rao A, Venkateswara Rao A, Pajonk GM (2007) Hydrophobic and physical properties of the ambient pressure dried silica aerogels with sodium silicate precursor using various surface modification agents. Appl Surf Sci 253(14):6032–6040
Bhagat S, Kim YH (2008) Superhydrophobic silica aerogel powders with simultaneous surface modification, solvent exchange and sodium ion removal from hydrogels. Microporous Mesoporous Mater 112(1–3):504–509
Nicholas L, Chakkaravarthy C, Bang A, Chariklia Sotiriou-L (2014) Cocoon-in-web-like superhydrophobic aerogels from hydrophilic polyurea and use in environmental remediation. ACS Appl Mater Interfaces 6(9):6872–6882
Kim CY, Lee JK, Kim BI (2008) Synthesis and pore analysis of aerogel-glass fiber composites by ambient drying method. Colloid Surf A Physicochem Eng Asp 313–314:179–182
Karout A, Buisson P, Perrard A (2005) Shaping and mechanical reinforcement of silica aerogel biocatalysts with ceramic fiber felts. J Sol-Gel Sci Technol 36(2):163–171
Li J, Cao JG, Li H (2012) One-step synthesis of hydrophobic silica aerogel viainsitu surface modification. Mater Lett 87(15):146–149
Ouyang ZH, Wu L, Li KB et al. (2005) Surface modification of nano-SiO2 in gas phase. Chem Ind Eng Progress 11(24):1265–1268.
Funding
This study was financially supported by the Joint Fund of the National Natural Science Foundation of China and China Academy of Engineering Physics (NSAF) (No. 11076010).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
He, F., Cheng, J., Wu, JY. et al. Dual modification of silica aerogel monoliths. J Sol-Gel Sci Technol 90, 323–329 (2019). https://doi.org/10.1007/s10971-019-04954-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-019-04954-z