Abstract
Immunoassays employed to measure C-peptide in rat serum neither have high sensitivity nor good range. Hence, the aim of this work was to develop a sensitive and specific user friendly immunoradiometric assay (IRMA) utilizing in-house magnetizable cellulose particles for quantification of C-peptide in rat serum. For development of IRMA, detector antibody (M1*) was prepared by chloramine-T method using 125I and capture antibody (SM2) was prepared by carbonyldiimidazole (CDI) activation method. The matched pair M1*-SM2 yielded an assay with adequate sensitivity (0.24 ng/ml) and range (0–20 ng/ml). C-peptide concentrations in rat serum samples (n = 30) analyzed using the developed assay ranged between 0.3 and 0.54 ng/ml. The reported C-peptide range can be used as a reference in impending diabetic research carried out using rat models.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is a predominantly concerned health challenge and becoming a major cause of death around the world, affecting the large population both in developed and developing countries [1]. According to the World Health Organization (WHO), the prevalence of DM is increasing. In the year 2019, it was the ninth leading cause of mortality, and over 1.56 million deaths were reported [2]. DM is a metabolic disorder that occurs because of deficient insulin production or the result of ineffective use of insulin from the body [3]. C-peptide is considered a reliable indicator of endogenous insulin synthesis since they are co-released into circulation in equimolar concentrations [4]. Unlike other species, rats produce two preproinsulins, which undergo cleavage and form rat proinsulin I and rat proinsulin II. Though rat C-peptide is produced in isoforms, rat C-peptide I and rat C-peptide II differ by only two amino acids at the 8th and 17th position [5]. The most appropriate measure of endogenous insulin secretion and apparent β-cell function is based on analysis of C-peptide using a standard method [6]. Measurement of C-peptide reflects the pancreatic insulin secretion rate more accurately than insulin itself. Also, C-peptide concentrations are independent on exogenous insulin administration and not subject to interference from insulin auto antibodies induced by therapy with insulin [7]. C-peptide measurements are preferable over insulin measurement because of lack of hepatic extraction, and slower metabolic clearance rate [8]. Comparison studies by Wszola et al. [9] demonstrated the superiority of rat models in comparison to mice models for diabetes research. A variety of immunoassay methods are presently employed to measure C-peptide in rat serum [10, 11]. However, an assay with the required sensitivity and good range is collectively not available. In addition, there is a scarcity of heterologous assays for C-peptide measurement in rats and mice separately. Hence, a sensitive and user-friendly immunoradiometric assay (IRMA) is developed with minimum assay steps. Apart from this, no assays were reported for rat C-peptide measurement using magnetizable cellulose particles as a separation system, which have subsequent criteria for application in immunoassays. Antibody coupled magnetizable cellulose particles form a stable solid phase and enable separation in short times using moderate magnetic fields [12, 13]. Kadwad, et al. [14] reported that the magnetizable cellulose particles with average size of 3 µm have a higher tendency to sediment in the moderate magnetic field. Rashmi, et al. [12] have shown that magnetizable cellulose particles can be employed for the development of the human C-peptide IRMA. Magnetic particles are accepted as suitable and effective separation system in chemiluminescence enzyme immunoassay [15], immunoradiometric assay [16], competitive enzyme assay [17] and radioimmunoassay [18, 19]. The selection of a separation system in IRMA is a very crucial step in assay development where the limitation of the PEG separation system in IRMA increases the popularity of optimally sized magnetizable cellulose particles (solid-phase) as a separation system. This work aims to develop a sensitive and specific user friendly IRMA utilizing in-house magnetizable cellulose particles for quantification of C-peptide in rat serum.
Materials
Chemicals
Synthetic rat C-peptide was purchased of purity ≥ 95%, purified by HPLC was obtained from Cellmano biotech limited, China. Sodium iodide (Na125I) was provided by the Bhabha Atomic Research Centre (BARC), Mumbai, India. Chloramine-T, sodium metabisulphite (MBS), potassium iodide, 1, 1’-carbonyldiimidazole (CDI), and Sephadex G-75 were procured from Sigma Aldrich Company, USA. Bovine serum albumin (BSA), human serum, bovine serum and horse serum are from Hi-Media, India. Human serum, bovine serum, rat serum and horse serum were treated with charcoal to remove protein components. Magnetizable cellulose particles were in-house produced in Board of Radiation and Isotope Technology (BRIT), Navi Mumbai, [Indian Patent No: 193445]. Monoclonal antibodies clones (CC-34 and CII-11) were procured from MyBioSource, USA. Trehalose was from Lobo chemicals, India. Multi-well gamma counter was purchased from Stratec Biomedicals Systems, Germany and single-well gamma counter is from Electronics Corporation of India Limited, India. Electrophoresis Unit is of Biotech, India and Whatman paper strip is from Whatman Pvt Ltd., England. Commercial rat sandwich ELISA kit was procured from Elabscience, China [E-EL-R3004 Rat C-P (C-peptide) ELISA kit 96 T].
Animals
Wister albino rats (weight = 150–200 g, n = 30) were purchased from the authorized dealer Adita Biosys private limited, Tumkur, and Institutional Animal Ethics Committee (IAEC) (SCP/IAEC/F150/P167/2019 dated 27.12.2019) provided approval for the work. Rats were housed in a separate room by accommodating three rats per cage and acclimatized to 12 h light–dark cycle for a week before blood collection. The experiment was conducted in the institution's animal house with all the precautions and temperature was maintained to 25 ± 5 °C with humidity < 70%.
Experimental
Radio-iodination of anti-rat C-peptide monoclonal antibody
Radio-iodination was carried out by chloramine-T method with slight modifications [20]. The column was prepared priorly for the purification of the radio-iodinated reaction mixtures of detector antibodies M1 and M2. The purification was carried out by column chromatography using Sephadex G 75, which was soaked overnight in 0.05 M phosphate buffer, pH 7.4, and loaded onto the column. The Sephadex column was allowed to settle down for 2 h, saturated with BSA (5 mg/ml) and eluted using 0.05 M phosphate buffer pH 7.4 as the elution buffer. The radio-iodination procedure followed is given in Table 1. The reaction mixture was loaded to the Sephadex G-75 column for purification and eluted with elution buffer at a constant flow rate (6 drops/minute). Purified fraction was collected in the polystyrene tube containing 1 ml of 0.05 M phosphate buffer pH 7.4 with 1% BSA and counted in a single-well gamma counter. Here, fractions with the highest counts were pooled and diluted using the tracer dilution buffer (0.05 M phosphate buffer with 0.2% BSA). Counts were adjusted to 70,000 counts per minute (cpm), the tracer was dispensed (1 ml) and stored at −20 °C for further use.
The prepared tracer was characterized for its specific activity, Radiochemical purity (RCP) and immunoreactivity. The reaction yield and specific activity of the radio-iodinated reaction mixture was determined by paper chromatography. Around 100 µl of reaction mixture was diluted with 400 µl of 0.05 M phosphate buffer pH 7.4 and 100 µl of potassium iodide. The reaction mixture was spotted on the pre-equilibrated Whatman paper strip and allowed to run for 1 h at 240 V in the electrophoresis bath. Mobility of free iodide was confirmed by streaking the paper strip with palladous chloride (PdCl2) solution. During electrophoresis, fastest moving I¯ (iodide) migrated to about 20 cm from the point of spotting. As a result, two radioactive peaks were obtained; radiolabeled antibody and the free radio-iodide. The percentage radioactivity associated with each peak was calculated and percentage yield was determined as follows:
Specific activity is the activity per unit mass and is usually expressed in mCi/mg or µCi/µg. Specific activity was calculated from the percentage yield obtained in the paper electrophoresis method as follows:
RCP is the fraction of the total radioactivity associated with the desired chemical form (in the present case antibody) of interest. The air-dried strip was cut carefully to 1 cm length and counted in a multi-well gamma counter. The RCP of the purified fraction of desired radio-iodinated antibodies was calculated using the paper electrophoresis technique as described by Manupriya et al. [21].
Preparation of antibody coupled magnetizable cellulose particles
Magnetizable cellulose particles were prepared in-house by the process of wet grinding method [14] and found to be suitable for the development of an IRMA and RIA assay system. The coupling of magnetizable cellulose particles with monoclonal antibodies was done by CDI activation method with minor modifications. One gram of magnetizable cellulose particles was used to couple with monoclonal antibodies for IRMA. The particles were washed with double-distilled water and acetone. After the wash, particles were activated with the addition of 500 mg CDI dissolved in acetone. The activated magnetic particles were washed with acetone by placing on magnet block followed by a thorough wash with 0.1 M bicarbonate buffer pH 8.6 until the pungent smell of acetone goes off. Washed magnetic particles were divided into 500 mg each in 0.1 M bicarbonate buffer pH 8.6 and the two monoclonal antibodies (M1 and M2) were added and incubated for overnight under shaking conditions. After incubation, particles were washed alternately with 0.1 M sodium acetate buffer, (pH 4) and 0.1 M bicarbonate buffer, (pH 8.6). Subsequently particles were washed with 0.05 M phosphate buffer pH 7.4 and assay buffer (0.05 M phosphate buffer containing 0.2% BSA and 0.1% tween-20). Finally, the coupled antibody-magnetizable cellulose particles (solid-phases SM1 and SM2) were suspended in assay buffer and stored at 4 °C for further use.
Identification of capture-detector antibody matched pair
The following assay procedure of IRMA was carried out to determine the optimum assay reagents. Rat C-peptide standards 0, 0.625, and 20 ng/mL were used for the standardization of the initial assay. In each tube, 100 µl of detector antibody (M1* and M2*) was mixed with 200 µl of each standard in duplicates followed by the addition of 100 µl of solid phases (SM1 and SM2). Tubes were vortexed well and incubated for 3 h with continuous shaking. After incubation, the tubes were washed with 2 ml of assay wash buffer (0.05 M phosphate buffer pH 7.4, 0.2% BSA, and 0.1% tween 20), vortexed well, and kept on a magnetic rack for 20 min. The washing step was repeated after decanting the supernatant. The radioactivity associated with the pellet was counted using the gamma counter and the percentage binding (% B/T) was calculated.
Standard preparation
A stock of Rat C-peptide standard 1 mg/ml was prepared in the 0.05 M phosphate buffer pH 7.4 containing 0.5% trehalose. Working standard of 0, 0.625, 1.25, 2.5, 5, and 20 ng/ml, and controls at two ends 0.5 and 10 ng/ml were prepared using horse serum containing 0.5% trehalose and 0.1% sodium azide as the matrix. Aliquots of 0.5 ml of rat C-peptide standards and controls were dispensed, lyophilized, and stored at 4 °C for further use.
Optimization of assay reagents
The different parameters for optimizing the assay, viz, tracer counts, titer of solid-phase, sample volume, incubation temperature and incubation time were studied. The tracer counts of 3.5 × 105, 7 × 105 and 14 × 105 cpm/ml and sample volume of 25, 50 100, and 200 µl were tested. Solid-phase at a titer of 1:2, 1:4, and 1:6 were tested to find the suitable working concentration of solid-phase for the assay. Furthermore, assay performance was tested at various incubation times (2, 3, 4 h, and overnight) and at varied temperature (37 °C, room temperature, and 4 °C) to obtain an IRMA with adequate sensitivity and stability.
IRMA procedure
To establish a reliable assay, 100 µl of rat C-peptide standards, 100 µl of solid-phase (SM2), and 100 µl of detector antibody (M1*) were added to polystyrene tubes in duplicates. The assay mixture was vortexed thoroughly and incubated overnight with constant gentle shaking. The assay was separated by adding 2 ml wash buffer (0.05 M phosphate buffer, 0.2% BSA and 0.1% tween-20) and placing the tubes on a magnetic rack for 20 min. After the separation step, the supernatant was decanted and the washing step was repeated. The counts associated with solid-phase were counted in the multi-well NaI(Tl) gamma counter. The standard curve was plotted using log–log graph with the % B/T on the y-axis and the concentration of rat C-peptide on the x-axis. Unknown C-peptide concentrations in rat serum samples were estimated from the standard curve.
The high dose hook effect was evaluated by running a developed IRMA assay with rat C-peptide standard concentrations 0, 0.625, 1.25, 5, 20, 40, 80 and 160 ng/ml. After separation, the standard curve was plotted using obtained percentage binding (% B/T) and a fall in the standard curve at certain rat C-peptide concentration was noted.
The developed assay was validated for its quality control parameters, viz, recovery, intra-assay and inter-assay variation, sensitivity, dilution linearity, parallelism, high dose hook effect and reagent stability.
Recovery, intra-assay and inter-assay variation
Recovery tubes were set up by spiking the sample with a known concentration of the standard. The native sample and the spiked sample were assayed and recovery was calculated as follows:
Intra-assay variation was determined by setting up ten replicates of rat serum sample in a single assay. Inter-assay variation was determined by setting up rat serum sample replicates in 5 different assays. Mean and SD were recorded and the coefficient of variance was calculated using the formula
Sensitivity of the assay
The sensitivity of the assay was statistically calculated by setting up replicates of zero standards and expressed as mean + 2SD.
Dilution linearity and parallelism
Dilution linearity of the assay was analyzed as follows, the pooled rat serum was spiked with 10 ng/ml of synthetic rat C-peptide and it was further diluted to 1:2 and 1:4. The IRMA was performed by considering spiked serum samples (10 ng/ml), 1:2 and 1:4 diluted serums as unknown samples. Linearity percentage was calculated using the formula.
To analyze the parallelism of the developed rat C-peptide IRMA, three rat serum samples that displayed the highest C-peptide concentrations were diluted to a 1:2 ratio and estimated in the optimized IRMA as unknown samples. The percentage of obtained C-peptide concentration to expected C-peptide concentration was calculated.
Stability of the reagents
Stability of the assay reagents such as solid-phase, detector antibody, and standards were determined monthly using the developed assay with fresh tracer prepared each month and rest of the reagents from same batch.
Sample collection and analysis
Rat blood samples (n = 30) were collected by retro-orbital method from the overnight fasted Wister albino rats of both genders and kept at room temperature for an hour. Serum was separated by centrifugation at 3000 rpm for 15 min and stored at −80 °C until analysis. Analysis of serum samples was carried out by using an optimized IRMA assay procedure. The C-peptide concentration obtained by developed IRMA was compared with a commercial rat sandwich ELISA kit (Elabscience, China).
Data analysis
The data was analyzed and illustrations were drawn using Microsoft word 2019 and Originpro 9, USA www.originlab.com
Results and discussion
Characterization of capture antibody and detector antibody
In the radio-iodination process, chloramine-T is used as an oxidizing agent to label monoclonal antibody with 125I which is a detector antibody in the IRMA assay. The reagent concentrations were optimized to 10 µg of chloramine-T and 20 µg of MBS to target the specific activity of 14 µCi/µg by the simple, reproducible and inexpensive chloramine-T method. Labeled protein was retained at the point of spotting and free iodide moved towards the cathode. PdCl2 reacts with free iodide and gives yellow color at the free iodide area. The elution pattern obtained after purification of the reaction mixture resulted in two peaks. The first peak in Fig. 1a and c are labeled monoclonal antibody whereas second peak in these are of free iodide. Radio-iodination yield of detector antibody, M1* and M2* was 75.32% and 66.32% with specific activity 10.83 µCi/µg and 9.53 µCi/µg respectively. The RCP of the purified pooled fraction was determined by paper electrophoresis which was > 90%. Radio-iodination results suggested that though the radio iodination yield was slightly high for M1* compared to M2* but the determined RCP was found to be almost similar.
Radio-iodination of anti-rat C-peptide monoclonal antibodies, M1 and M2: a. Elution pattern of M1* fractions purified by Sephadex-G 75 column, b. Determined RCP of M1* purified fraction by paper electrophoresis on the day of radio iodination, c. Elution pattern of M2* fractions purified by Sephadex-G 75 column, d. RCP of purified fractions of M2*was determined by paper electrophoresis on the day of radio iodination. M1* = radio iodinated M1, M2* = radio iodinated M2
Detector antibody selection was based on its performance as a matched pair, here mostly the reaction with solid-phase play an important role. The detector antibodies were tested against both the solid-phases, SM1 and SM2, and examined for a compatible matched pair. The selection was based on the sensitivity index, non-specific binding, and maximum binding at a higher standard concentration. It is analyzed by cross matching the solid phase with detector antibodies as follows M1*-SM2 and M2*-SM1. The M1*-SM2 combination had a greater sensitivity index of 3.61% than the M2*-SM1 combination (Table 2), hence, it was recommended as the best-matched pair for the IRMA system.
Assay optimization and assay parameters
Rat C-peptide at concentrations of 0, 0.625, 1.25, 2.5, 5, and 20 ng/ml were used in the optimized assay technique. For higher concentrations (80 ng/ml) hook effect was seen. Hence, these lower concentrations were fixed for the assay.
In comparison to 3.5 × 105 and 14 × 105 cpm/ml, the detector antibody count of 7 × 105 cpm/ml was well matched for the assay.
The assay's maximum percent binding at the highest concentration of 20 ng/ml was found to be slightly higher for solid-phase dilution 1:2 (28%) than 1:4 (26.4%). However, 1:4 solid-phase dilutions was an appealing option as slight variation does not affect the assay performance and also it consumes the least amount of reagent.
For assay system optimization, different sample volumes like 25, 50, 100 and 200 µl was tested. Of these, 100 µl was found to be more suitable because it gave the desired maximum binding and NSB in the developed IRMA assay. Hence, sample volume of 100 µl was selected for the assay.
Maximum percent binding (26%) was observed for the assay incubated for 3 h with shaking or overnight with shaking. However, there was an increase in sensitivity from 0.46 ng/ml to 0.24 ng/ml when incubated overnight with shaking. Hence, overnight incubation with shaking was chosen for the developed IRMA.
The Shewhart chart was used to fix the concentration of controls; control 1 (0.50 ± 0.06 ng/ml) and control 2 (10.4 ± 2.4 ng/ml) for the assay. In all test trials, the control values should always fall within the specified range for sample concentration acceptance.
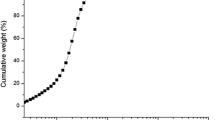
A typical standard curve obtained by an optimized rat C-peptide IRMA is depicted in Fig. 2a.
Experimental scheme of magnetic particle-based rat C-peptide IRMA and stability of its reagents: a. Illustration of typical standard curve of developed rat C-peptide IRMA using known rat C-peptide concentrations, radioactive detector antibody, and magnetic particles based capture antibody-the measured radioactivity facilitate to estimate unknown samples, b. Stability of rat C-peptide standards for 6 months c. Stability of M1* determined for 60 days (frozen stored at -20 °C), d. Stability of solid-phase (SM2) of IRMA stored at 4 °C
Recovery studies in an immunoassay are meant to evaluate the ability of the assay in measuring the protein present in serum samples [22]. The performed assay is concluded to be accurate and acceptable when the observed value comes under the range of 80–120% concerning the expected value [23]. In the present assay, the obtained recovery range is 93–109% for tested rat serum samples (n = 4) (Table 3).
In rat C-peptide IRMA, intra-assay variation for two samples were 4.4 and 7.1%. The intra-assay variation of up to 15% is acceptable for immunoassays [24] The values obtained for inter-assay variation were 8.1 and 14.3% which falls under the acceptable criteria of < 15% for inter-assay variation [25].
The statistically calculated sensitivity of the developed rat C-peptide IRMA was 0.24 ng/ml.
Dilution linearity and parallelism studies of the assay were observed within the acceptable range as given in Tables 4 and 5.
Stability of the assay reagents and rat serum sample analysis
The stability studies showed that the detector antibody was stable for 45 days at −20 °C from the day of iodination. Therefore, a reduction in Bmax from 26% (on the day of radio-iodination) to 19% (60th day) was observed at 20 ng/ml (Fig. 2c).
The shelf life of the solid phase was observed to be more than two years at 4 °C (Fig. 2d).
The stability of standards was satisfactory up to 6 months. The stability of the C-peptide standard was initially observed for only 15 days in plain 0.05 M phosphate buffer pH = 7.4. So, an effort was made to improve the stability of rat C-peptide standards using treated human, bovine, rat and horse serum as a matrix. Of these, horse serum was found to be a good matrix for rat C peptide standard preparation. Further C-peptide stability was enhanced up to 6 months by adding a protein stabilizer (0.5% trehalose) and finally, lyophilized and stored at 4 °C.
The unknown concentration of C-peptide in rat serum sample was estimated by the obtained typical IRMA standard curve Fig. 2a. Obtained results against the standard curve suggested that normal concentrations of rat C-peptide ranged between 0.15 to 0.48 ng/ml. Developed IRMA assay for rat C-peptide was calibrated against commercially available kit (Elabscience, China), and the correlation equation of y = 0.9949x + 0.0068 with R2 = 0.9657 was obtained (Fig. 3).
Conclusions
Present study is designed for the estimation of the normal C-peptide concentration in rat serum and examined C-peptide concentration range can be used as a reference in imminent research. The developed immuno-radiometric assay can also provide a sensitive measure of C-peptide in diabetic research. Rat C-peptide IRMA has dominance over other available methods by a wide standard range (0 to 20 ng/ml), economical solid-phase separation system, and prolonged standard and solid-phase stability. An attained sensitivity of 0.24 ng/ml made the developed assay adaptable in diabetes, insulinoma and hypoglycemia research.
References
Eluehike N, Onoagbe IO (2020) Preliminary nutrient determination and regeneration of pancreatic islet cells by extracts of spondiasmombin leave in streptozotocin-induced diabetic rats. Avicenna J Med Biochem 8(1):15–20
World Health Organization (WHO) (2021) Diabetes. Geneva: WHO. Available from: https://www.who.int/news-room/fact-sheets/detail/diabetes
Adienbo OM, Hart VO (2021) Antidiabetic effect of Persea Americana seed extract are mediated through enhanced insulin secretion, improved beta-cell function, and reduced insulin resistance in diabetic rats. Eur J Med Res 9(1):18–25
Banting FG, Best CH (1922) The internal secretion of the pancreas. J Lab Clin Med 7(5):256–271
Tager HS, Steiner DF (1972) Primary structures of the proinsulin connecting peptides of the rat and the horse. J Biol Chem 247(24):7935–7940
Horwitz DL, Rubenstein AHMD, Katz AI (1997) Quantitation of human pancreatic beta-cell Function by Immunoassay of C-Peptide in Urine. Diabetes 26(1):30–35
Schultess J, Duren CV, Martens M, Costa M, Llop T, Marti T, Eppinger M, Hausmann M, Krack W, Dhein J (2009) Diagnostic performance of the architect C-peptide immunoassay. Clin Chem Lab Med 47:834–841
Bonser AM, Garcia-Webb P, Harrison LC (1984) C-peptide measurement: methods and clinical utility. Crit Rev Clin Lab Sci 19(4):297–352
Wszola M, Klak M, Kosowska A, Tymicki G, Berman A, Adamiok-Ostrowska A, Olkowska-Truchanowicz J, Uhrynowska-Tyszkiewicz I, Kaminski A (2021) Streptozotocin-induced diabetes in a mouse model (balb/c) is not an effective model for research on transplantation procedures in the treatment of type 1 diabetes. Biomedicines 9(12):1790
Carlsson A, Hallgren IB, Johansson H, Sandler S (2010) Concomitant enzyme-linked immunosorbent assay measurements of rat insulin, rat C-peptide, and rat proinsulin from rat pancreatic islets: effects of prolonged exposure to different glucose concentrations. Endocrinology 151(10):5048–5052
Van Genderen FT, Gorus FK, Vermeulen I, Vekens EM, De Pauw PE, Pipeleers DG, Van Schravendijk C (2010) Development of a multipurpose time-resolved fluorescence immunoassay for rat insulin. Anal Biochem 404(1):8–13
Rasmi RR, Shenoy KB, Kadwad VB, Sarnaik J, Somashekarappa HM (2015) Application of novel magnetizable cellulose particles in the development of an immunoradiometric assay for C-peptide. J Radioanal Nucl Chem 304(3):1115–1122
Galkin OY, Besarab OB, Pysmenna MO, Gorshunov YV, Dugan OM (2018) Modern magnetic immunoassay: biophysical and biochemical aspects. Regul Mech Biosyst 9(1):47–55
Kadwad V, Jyotsna N, Sivaprasad N, Sinha P (1996) A method for preparation of magnetizable cellulose and its application in radioimmunoassay for T3 and T4. J Radioanal Nucl Chem 210(1):27–33
Wang X, Lin JM, Ying X (2007) Evaluation of carbohydrate antigen 50 in human serum using a magnetic particle-based chemiluminescence enzyme immunoassay. Anal Chim Acta 598(2):261–267
Prasad UV, Mohan RK, Samuel G, Harinarayan CV, Sivaprasad N, Venkatesh M (2012) Standardization of a two-site PTH immunoradiometric assay using various solid-phase formats. Indian J Med Res 136(6):963–970
Zhang B, Du D, Meng M, Eremin SA, Rybakov VB, He X, YinY XR (2014) A magnetic particle-based competitive enzyme immunoassay for rapid determination of ciprofloxacin: a potential method for the general detection of fluoroquinolones. Anal Lett 47(7):1134–1146
Gholve C, Kumarasamy J, Damle A, Kulkarni S, Venkatesh M, Banerjee S, Rajan MGR (2019) Comparison of serum thyroglobulin levels in differentiated thyroid cancer patients using In-house developed radioimmunoassay and immunoradiometric procedures. Indian J Clin Biochem 34(4):465–471
Rasmi RR, Kadwad VB, Sarnaik S, Shenoy KB (2020) Development of radioimmunoassay for estimation of C-peptide in human serum. J Radioanal Nucl Chem 327:923–928
Greenwood FC, Hunter WM, Glover JS (1963) The preparation of 131I-labelled human growth hormone of high specific activity. Biochem J 89:114–123
Manupriya BR, Paradkar S, Lathika PSL, Somashekarappa HM, Shenoy KB (2018) Optimization of reagent concentration for radioioination of rat C-peptide II in development of radioimmunoassay procedure for rats. Radiat Prot Environ 41(1):26–29
Ravisankar P, Navya CN, Pravallika D, Sri DN (2015) A review of step-by-step analytical method validation. IOSR J Pharm 5(10):7–19
Omar NAS, Fen YW, Ramli I, Sadrolhosseini AR, Abdullah J, Yusof NA, Kamil YM, Mahdi MA (2021) An optical sensor for dengue envelope proteins using polyamidoamine dendrimer biopolymer-based nanocomposite thin film: enhanced sensitivity, selectivity, and recovery studies. Polymers 13(5):762
Giltinan DM, Davidian M (1994) Assays for recombinant proteins: a problem in non-linear calibration. Stat Med 13(11):1165–1179
Ramljak S, Musholt PB, Schipper C, Flacke F, Sieber J, Borchert M, Forst T, Pfützner A (2013) The precision study: examining the inter-and intra-assay variability of replicate measurements of BGStar, iBGStar, and 12 other blood glucose monitors. Expert Opin Med Diagn 7(6):511–516
Acknowledgements
The author Manupriya B R is grateful to the University Grant Commission Basic Scientific Research (UGC-BSR) fellowship for extending financial support to carry out this work. The author is grateful to Dr. Karunakara Hedge, Department of Pharmacology, Srinivasa College of pharmacy, Mangaluru, India- 574143 for providing the animal house and assistance in blood collection from rats.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Manupriya, B.R., Paradkar, S., Ghodke, T.S. et al. Development of a magnetizable cellulose particle-based immunoradiometric assay for quantification of C-peptide in rat serum. J Radioanal Nucl Chem 332, 517–525 (2023). https://doi.org/10.1007/s10967-023-08796-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-08796-6