Abstract
The performance of LSC cocktails in ultra-sensitive applications was evaluated. Backgrounds from radioactive contaminations in commercially available and in-house developed liquid scintillation cocktails were measured and compared to the predicted background levels of the ultra-low background liquid scintillation counter. Through the ICP-MS assay of the cocktails and their constituents, potassium impurities in the surfactant component were identified as a significant source of background, potentially limiting the use of LSC counting in ultra-sensitive applications. This work lays the groundwork for future research towards ultrapure LSC cocktails for ultrasensitive LSC counting.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Liquid scintillation counting (LSC) is used in a wide variety of fields ranging from medical applications [1], environmental and non-proliferation monitoring [2, 3] to fundamental chemistry and physics research studies [4,5,6,7,8]. Organic liquid scintillator cocktails are composed of a scintillation solvent base, a primary fluorescent agent (fluor), and a secondary wavelength shifter [9]. Solvents are typically aromatic benzene derivatives, which are rich in \(\pi\)-electron conjugated systems. The light production in a liquid scintillator involves a sequence of various physical and chemical processes. Most of the energy deposited by ionizing radiation (\(\alpha\), \(\beta\)) passing through the scintillator media is converted by delocalized \(\pi\)-electrons of the scintillator base, resulting in the direct excitation of solvent molecules. Molecules in the excited states decay emitting photons, which can be received through a dipole-dipole interaction or absorption/reemission by the fluor. A secondary wavelength shifter is often added to further red-shift the emitted photon wavelength to match the sensitivity region of the light detector used by the LSC system, typically a photomultiplier tube (PMT).
Neat organic liquid scintillators are not miscible with water, due to the difference in molecular polarity. Most scintillator cocktails used in LSC employ surfactants, containing lypophilic and hydrophilic groups, to emulsify and stabilize aqueous and organic scintillator mixtures. A new water-based liquid scintillator (WbLS) [10], originally developed for deployment of kiloton scale particle detectors, is capable of loading water in high content (40%) in organic liquid scintillators with high optical transparency, chemical stability, and radiopurity, allowing its application in LSC as well. It should be noted that commercial liquid scintillation cocktails, designed for particle detection in aqueous solutions, are of similar chemical composition of WbLS.
The performance of liquid scintillation cocktails is evaluated based on parameters such as light yield, intrinsic backgrounds, quench resistance, stability over time, and sample load capacity, i.e. the maximum amount of aqueous sample that can be dissolved into the cocktail to form a homogeneous, optically transparent solution [11, 12]. Intrinsic radioactive impurities can limit the performance of commercially available LSC cocktails for ultra-low background applications. Radioactive decays from the cocktail components themselves can interfere with signatures of interest for low sample activities [13, 14]. Liquid scintillator cocktails are predominantly composed of carbon, one of the main background sources is represented by radiocarbon \(^{14}\)C (T\(_{1/2}\) = 5730 y), which cannot be chemically separated. A significant reduction of \(^{14}\)C background is obtained using petroleum-derived scintillators [15]. In petroleum, that sits underground for long periods, the cosmogenic production of \(^{14}\)C is minimized and most of the radiocarbon originally presented has already decayed after millions of years of storage. Another significant source of background in liquid organic scintillators is represented by naturally occurring primordial radionuclides, \(^{40}\)K, \(^{232}\)Th and \(^{238}\)U and their daughters. These radionuclides are commonly present in materials [16] and may be introduced during scintillator production, sample handling, transportation, and/or exposure to dust particulates [17]. As opposed to radiocarbon, impurities of \(^{40}\)K, \(^{232}\)Th and \(^{238}\)U in liquid organic scintillators can be significantly reduced using purification methods. Some examples of liquid organic scintillator purification procedures for ultra-sensitive applications are reported in [8, 13, 18,19,20]. Progenies in the lower portion of the \(^{232}\)Th and \(^{238}\)U chains oftentimes are not in secular equilibrium with the rest of the chain. When possible, pulse shape analysis can be applied to discriminate background contributions from \(\alpha\)-emitters in the chain. Radon found in air also contributes to elevated background in liquid scintillators.
An ultra-low background liquid scintillation counter (ULB LSC) was constructed at Pacific Northwest National Laboratory [21]. The ULB LSC is housed in the PNNL Shallow Underground Laboratory [22], which provides roughly 30 m water-equivalent reduction from cosmic-ray background [23]. Additionally, the ULB LSC was designed using the concepts from the development of the PNNL ultra-low background proportional counters [24]. A full description of the ULB LSC design can be found in [25], but aspects that are relevant to the liquid scintillation cocktail background studies will be discussed here. The ULB LSC is comprised of two PMTs that are arranged at 90\(^{\circ }\) to the samples, as opposed to the more traditional face-on configuration of commercial systems. A hollow ultra-pure copper light-guide, coated and polished for maximum light transmission, connects the face of each PMT to the sample holder. This geometry was chosen to provide the capability to shield each of the PMTs from each other as well as from the sample to mitigate intrinsic radioactivity present in the PMTs from contributing to the background. Additionally, optical simulations of the geometry were performed to ensure light-collection efficiency was maximized [26]. The internal detection configuration is then further shielded via layers of ultra-low background copper and various grades of lead. Finally, veto panels are used in anti-coincidence to eliminate events caused by any cosmic-ray contributions. A diagram of the system is presented in [25]. Additionally, the entire system is nitrogen purged to prevent Rn backgrounds. As the ULB LSC is more sensitive than typical commercial instrumentation used in above-ground laboratories, the radiopurity of the constituents of samples, such as the liquid scintillation cocktail, can limit the sensitivity of detection for low-level applications.
The background estimation for the ULB LSC from the construction materials is given in Table 1. The values for the various materials have been updated since the initial background budget published by Erchinger et al. [21]. Values were updated based upon recent assay work from rare-event search physics experiments [28,29,30,31,32]. The updated background budget is roughly 25\(\%\) higher than the previous estimations [21]. However, these improvements are still too low to account for the observed background rates from the ULB LSC. The target background range for the ULB LSC is 10–100 counts per day (cpd), which would provide 10-100x more sensitivity compared to commercial systems with a background rate of 1–10 counts per minute (cpm) depending on the location, scintillation cocktail, and sample matrix. Initial measurements using the ULB LSC observed 420 ± 50 cpd background [21] for a standard polyethylene scintillation vial with 20 mL of Ultima Gold LLT. While this still presents an improvement compared to commercial systems at roughly 0.3 cpm, this is an order of magnitude above the expected background. Given the intrinsic \(^{14}\)C,\(^{40}\)K, \(^{232}\)Th, and \(^{238}\)U content in liquid scintillation cocktails, the increased background is suspected to arise from the cocktails themselves. Therefore, to achieve the sensitivity improvements expected from the ULB LSC, a reduction of the LSC cocktail’s intrinsic background is necessary.
In this work, we have investigated traces of intrinsic radioactive impurities in both commercially-available and in-house developed LSC cocktails. Ultra-trace elemental analysis with Inductively Couple Plasma Mass Spectrometry (ICP-MS) was performed at PNNL, focusing on radionuclides \(^{40}\)K, \(^{232}\)Th and \(^{238}\)U. Potassium contaminations were identified as a dominant source of background. Concentrations of \(^{nat}\)K on the order of a few to tens of \(\mu\)g\(\cdot\)g\(^{-1}\), corresponding to activities of a fraction to a few Bq\(\cdot\)kg\(^{-1}\) from \(^{40}\)K, were measured. Impurities of Th and U were determined in the pg\(\cdot\)g\(^{-1}\) regime, corresponding to activity levels of \(\mu\)Bq\(\cdot\)kg\(^{-1}\). A comparison with high purity germanium (HPGe) measurements performed at Savannah River National Laboratory’s (SRNL) Underground Counting Facility (UCF) was also performed, showing agreement between the two techniques. Single constituents of the LSC cocktails were also investigated via ICP-MS. The surfactant components were identified as the major sources of potassium contaminations in the LSC cocktails. Potassium concentrations in the cocktails were measured orders of magnitude higher compared to thorium and uranium.
Experimental
Materials
Ultra-low background perfluoroalkoxy alkane (PFA) screw cap vials from Savillex (Eden Prairie, MN) were used for preparation of reagent solutions and sample dilutions for ICP-MS analysis. Polyethylene (PE) LSC vials (Perkin Elmer, Waltham, MA) were used for sub-sampling and first level dilutions of the samples. Fused silica crucibles (Technical Glass Products Inc, Painesville Twp., OH) were used for sample dry ash in a tube furnace. Optima grade nitric and hydrochloric acid (Fisher Scientific, Pittsburg, PA) and 18.2 M\(\Omega \cdot\)cm deionized water from a MilliQ system (Merk Millipore GmbH, Burlington, MA) were used for preliminary labware cleaning and preparation of reagent solutions. All labware underwent preliminary cleaning before use. PFA vials, fused quartz crucibles, and pipette tips were sonicated in a 2\(\%\)(v/v) Micro90TM (Cole-Parmer, Vernon Hills, IL) solution, rinsed in MilliQ water and leached in 3M HCl and 6M HNO\(_3\) solutions. LSC vials were cleaned in a 2\(\%\)(v/v) Micro90TM solution followed by thorough rinse in deionized water. Following cleaning, all vials and crucibles were validated: a small volume of 5\(\%\) nitric acid solution was pipetted into each vial/crucible, vials were recapped and shaken. PFA vials were heated at 80\(^\circ\)C for at least 12 h while LSC vials and crucibles were kept at room temperature for a few hours. After this period, PFA vials were cooled down to room temperature and solutions from all vials and crucibles were analyzed on an ICP-MS for the analytes of interest. Validations are performed to assure that measured background signals from the containers for the analytes of interest are within instrumental background. Any labware not meeting such requirement underwent additional cycles of cleaning and validation until signals were measured within instrumental background.
Standard solutions of \(^{229}\)Th and \(^{233}\)U (Oak Ridge National Laboratory, Nuclear Science and Technology Division, Nuclear Medicine Program, Oak Ridge, TN) were used for determinations of \(^{232}\)Th and \(^{238}\)U via isotope dilution method. A potassium standard solution (Inorganic Ventures, Christiansburg, VA) with natural isotopic abundance was used to generate external calibration curves for K quantitations. Vials used to store samples for HPGe measurements were acid cleaned with fuming nitric acid (Fisher ACS grade), rinsed with deionized water (18.2 M\(\Omega \cdot\)cm), and dried.
Samples
Samples of commercially-available liquid scintillation cocktails, listed in Table 2, were received from vendors for ICP-MS analysis. Single components constituting the cocktails were received and analyzed for Meridian Gold Star LT2 cocktail, National Diagnostics Ecoscint Ultra and H cocktails. Two samples of LSC cocktails developed and produced at Brookhaven National Laboratory (Upton, NY) were also investigated.
Samples of Ultima Gold uLLT, Ultima Gold AB, and Ecoscint Ultra were also independently purchased (different production lots) for HPGe analyses at SRNL. Subsamples of the same production batch of the BNL cocktails were provided to PNNL and SRNL for ICP-MS and HPGe analyses respectively.
ICP-MS determinations
A class 10,000 cleanroom and a laminar flow hood providing a class 10 environment at PNNL were used for sample preparation and analyses. Determinations of K, Th and U were performed using an Agilent 8900 triple quadrupole ICP-MS (Agilent Technologies, Santa Clara, CA), equipped with an integrated autosampler, a microflow PFA nebulizer and a quartz double pass spray chamber. Thorium and uranium determinations were performed operating the instrument in single quadrupole, no-gas mode. Plasma, ion optics and mass analyzer parameters were optimized based on the instrumental response from a standard solution from Agilent containing 0.1 ng\(\cdot\)mL\(^{-1}\) of Li, Mg, Co, Y, Ce and Tl. The instrumental response from Tl was used as a reference signal to maximize the signal/noise ratio in the high m/z range. Oxides were monitored and kept below or around 2\(\%\) based on the m/z=156 and m/z = 140 ratio (CeO\(^+\)/Ce\(^+\)) from Ce in the standard solution. Potassium determinations were performed in cool plasma with NH\(_3\) reaction mode. Instrumental parameters were optimized based on the instrumental response from a 1 ng\(\cdot\)g\(^{-1}\) K standard solution, in house diluted from a stock solution. An acquisition method including three replicates and ten sweeps per measurement was used. Acquisition times were set based on the expected signal, in order to maximize the instrumental precision by improving counting statistics. For the quantitation of \(^{232}\)Th and \(^{238}\)U, samples were spiked with a known amount of \(^{229}\)Th and \(^{233}\)U tracers prior to digestion. Concentrations were calculated using isotope dilution methods, using Eq. 1:
where \(A_{analyte}\) and \(A_{tracer}\) are the instrumental responses for the analyte and the tracer, respectively, and \(C_{tracer}\) is the spiked concentration of tracer in the sample. Quantitation of \(^{nat}\)K was performed through an external calibration curve, using potassium standards with natural isotopic composition. The signal from the most abundant isotope, \(^{39}\)K, was used as a reference for measurements. Potassium natural isotopic abundance was assumed in the samples. It is worth pointing out that ICP-MS does not detect radiation from decays, it measures ions. Results are obtained in concentrations (e.g., ppt or pg\(\cdot\)g\(^{-1}\) of sample). Corresponding activities (e.g., \(\mu\)Bq\(\cdot\)kg\(^{-1}\)) are calculated based on the specific activity and isotopic abundance of the studied radionuclides. For interest in this work, 1 pg\(\cdot\)g\(^{-1}\) \(^{238}\)U corresponds to 12.4 \(\mu\)Bq\(\cdot\)kg\(^{-1}\), 1 pg\(\cdot\)g\(^{-1}\) \(^{232}\)Th corresponds to 4.06 \(\mu\)Bq\(\cdot\)kg\(^{-1}\) and 1 \(\mu\)g.g\(^{-1}\) \(^{nat}\)K corresponds to 30.5 mBq\(\cdot\)kg\(^{-1}\) from \(^{40}\)K (0.01\(\%\) natural abundance).
All samples were prepared and analyzed in triplicate. Process blanks (n = 3) were prepared for each analysis. Signals from samples were process blank subtracted. Results are reported as the average and standard deviation of three independent replicates. Samples underwent a dry ash digestion in fused quartz crucibles in a tube furnace (Thermcraft, Winston-Salem, NC) for Th and U assays. Portions of ca. 50-100 mg of sample were digested at 800\(^\circ\)C under a 4 L\(\cdot\)min\(^{-1}\) air flow. A known amount, on the order of 100-200 fg, of \(^{229}\)Th and \(^{233}\)U radiotracers was added into each sample replicate and process blank prior digestion. Sample and radiotracer masses were determined gravimetrically. In order to determine potassium recoveries, some of the sample replicates were spiked with a known amount of \(^{nat}\)K from a standard solution, ca. 500 ng, corresponding to a concentration of the order of 5 \(\mu\)g\(\cdot\)g\(^{-1}\) in the cocktail, prior digestion. After full digestion, any residual ash in the crucibles was redissolved in 8M HNO\(_3\) on a hotplate at 170\(^\circ\)C, followed by boiling off of the 8M HNO\(_3\) solution and reconstitution of the samples in a 2\(\%\) HNO\(_3\) solution for ICP-MS analysis. Thorium and uranium recoveries were measured > 50\(\%\) in all samples, whereas potassium recoveries were very low low (< 50\(\%\)) for most of the samples. Further investigations about potassium losses were considered beyond the purpose of this work and were not performed. Given the levels of expected concentrations (\(\sim\) \(\mu\)g\(\cdot\)g\(^{-1}\), determined from preliminary dry ash analyses), high dilutions or liquid liquid extractions in 2\(\%\) HNO\(_3\) solution were performed, depending on sample water miscibility, for potassium determinations. Dilution factors of the order of 10,000-100,000 were required prior to ICP-MS injection.
HPGe measurements
Radioactive contributions from \(^{40}\)K were measured using HPGe in the UCF at SRNL in five of the samples listed in Sect. 2.2: two LSC cocktails developed in-house at BNL, Ultima Gold uLLT and Ultima Gold AB from Perkin Elmer, Ecoscint Ultra from National Diagnostics. Regarding BNL LSC cocktails, the same production batch analyzed at PNNL via ICP-MS was measured at SRNL. Perkin Elmer and National Diagnostics samples were instead different, independently purchased, production lots compared to the samples analyzed at PNNL. For the HPGe measurement, samples were prepared in an ISO-17025 accredited low-level radiochemistry laboratory and placed in acid rinsed vials, sealed, and bagged for placement in UCF HPGe detectors. The detectors are surrounded by a layer of ultra-pure copper, an outer layer of 10 cm minimum unsealed, low-background lead housed in the UCF vault located 14.6 m below ground level and constructed from pre-WWII USS Antietam (CG-54) steel and backfilled with layers of borated cement, specular hematite, and clay for a water overburden equivalency of 31.7 m [33]. Two types of HPGe detectors were used, a 4 mL Well (ORTEC) and a GMX n-type (Canberra). Both are set up so that radon daughters are driven out of the sample chamber using the liquid nitrogen exhaust. Gamma spectra acquisition began several days after sample placement into the detector chambers in order to remove spectral contributions from radon daughters. Samples were counted for a minimum of one week and results from each detector were corrected for background and detector energy efficiency with the final results given in Fig. 2 as the average of both \(^{40}\)K determinations.
Results
Table 3 reports the levels of potassium, thorium and uranium measured in the LSC cocktails via ICP-MS elemental analysis. Concentrations (in pg\(\cdot\)g\(^{-1}\) of cocktails) of \(^{nat}\)K, \(^{232}\)Th and \(^{238}\)U were determined via ICP-MS. Corresponding activities, in mBq\(\cdot\)kg or \(\mu\)Bq\(\cdot\)kg\(^{-1}\) of cocktail, were determined based on radionuclide specific activities. Concentrations of \(^{nat}\)K were converted to the corresponding activity of \(^{40}\)K assuming natural isotopic abundance of potassium in the samples. Results for each sample are reported as the average and standard deviation of three independent replicates.
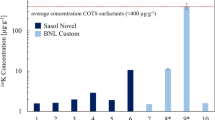
Figure 1 shows results in terms of decays\(\cdot\)day\(^{-1}\cdot\)sample\(^{-1}\) from \(^{40}\)K, \(^{232}\)Th, and \(^{238}\)U in a 10 mL volume (typically used for sample analysis) of each cocktail. Each value is reported as the average and standard deviation of three independent replicates. Error bars are hidden under the marker where not visible. The red dashed line in the plot is the level of the predicted background in the ULB LSC (Section 1). For all cocktails, background from \(^{232}\)Th and \(^{238}\)U is significantly below the predicted baseline background for the LSC system. Decays were calculated only accounting for the parent radionuclide in the chain. Even accounting for decays from the full decay chain, assuming secular equilibrium, the resulting background would be below the LSC counter intrinsic background. Impurities of \(^{232}\)Th and \(^{238}\)U in the cocktails, therefore, do not represent a concern for ultra-sensitive measurements. On the contrary, the background potentially generated by potassium contaminant in the cocktails is significantly above the system predicted background for all the cocktails. The content of potassium in the scintillation cocktails, thus, limits the sensitivity of the system and may be a concern for ULB measurements.
Potassium measurements in the cocktails performed via ICP-MS at PNNL were compared with results obtained at SRNL via HPGe counting. Figure 2 reports results, in mBq of \(^{40}\)K per kg of cocktail, obtained at PNNL and SRNL for five of the investigated cocktails. The agreement between data obtained with the two techniques represents a further validation for both methods. A slight discrepancy, of a factor of \(\sim\)3, was observed for the Ecoscint Ultra cocktail from National Diagnostics. The discrepancy may be attributed to different production lots, as the samples were independently obtained by PNNL (provided by the manufacturer) and SRNL (purchased).
The analyses of the commercially available and in-house developed LSC cocktails for traces of radioactive impurities revealed that intrinsic contaminations of potassium may be a significant contributor for radioactive background increase, limiting sensitivity in ULB LSC applications. The dominant vectors of potassium in the cocktails were investigated through ICP-MS elemental analysis of cocktail single constituents. Surfactant components from two of the investigated cocktails were analyzed. Samples of the six single surfactants utilized in the Ecoscint H and Ecoscint Ultra LSC cocktails production from National Diagnostics were received as blind samples, as listed in Table 4. It was only revealed that surfactants 1, 2 and 3 are used as a mixture in Ecoscint H; a mixture of surfactants 4, 5 and 6 is a constituent of Ecoscint Ultra. Neither the nature of each surfactant nor the relative composition of each surfactant mixture were revealed. Concentrations of \(^{nat}\)K measured in the single surfactants, along with those measured in the LSC cocktails are also reported in Table 4. Considering the content of the surfactant mixture in the cocktails (\(\sim\)20-30\(\%\)) reported in the MSDSs, the potassium amount determined in the surfactants accounts for the potassium content measured in the cocktails.
Single Gold Star LT2 LSC cocktail constituents were also received from Meridian. The surfactant component of this cocktail is a mixture of three Nonyl-PhenolEthoxylates (NPEs). All three NPEs were analyzed for potassium via ICP-MS. Results, along with the K content in the cocktail and the approximate content of each NPE in the cocktail, are reported in Table 5. Considering the content of the NPEs in the cocktail (20–30\(\%\)), and the measured potassium concentrations, again the potassium amount measured in the surfactant components accounts for the total potassium content in the cocktail.
It is worth noting that some surfactants, including NPEs, are typically synthesized using a potassium based catalyst, which validates results from this work.
Conclusions
Intrinsic radioactive backgrounds in commercially available and in-house developed LSC cocktails were determined from trace analysis of naturally occurring radionuclides \(^{nat}\)K, \(^{232}\)Th, and \(^{238}\)U. Backgrounds in terms of decays\(\cdot\)day\(^{-1}\) from a volume of 10 mL sample were estimated using radionuclides specific activities, and compared to the predicted baseline background of the ULB LSC at PNNL. As opposed to Th and U, intrinsic impurities of K were determined as a concerning source of background contribution, potentially limiting sensitivity in ULB LSC applications. Potassium was measured in the \(\sim\) \(\mu\)g\(\cdot\)g\(^{-1}\) regime in all the investigated cocktails, translating to activities ranging from \(\sim\)100 to \(\sim\)3000 mBq\(\cdot\)kg\(^{-1}\) of cocktail from the radioactive isotope \(^{40}\)K (0.01\(\%\)) of naturally occurring potassium. Agreement between data obtained via ICP-MS at PNNL and via HPGe at SRNL provides a validation of the results. In a 10 mL portion of liquid scintillator cocktail, background would range from a hundred to a few thousands of decays per day, orders of magnitude above the estimated baseline background level of the ULB LSC at PNNL (12 ± 1 counts\(\cdot\)day\(^{-1}\)). Although only a fraction < 100\(\%\) of total decays will actually generate counts in the ULB LSC, the high \(^{40}\)K content will affect the detection limits of the instrument.
The primary source of potassium impurities in the cocktails were investigated and identified as the surfactants, typically produced using K-based catalysts. The surfactant component in the LSC cocktails is necessary for the analysis of aqueous samples. It stably emulsifies aqueous phases in the organic scintillation mixtures, which are otherwise immiscible. This work provides key information about intrinsic backgrounds in LSC cocktails for ultra-sensitive applications. The results of this study open the way to future research and development for the production of high-purity LSC cocktails for ultra-sensitive applications. Further investigation of potential purification processes of LSC cocktails and surfactants, alternative surfactant production, and the use of alternative low-K surfactants in LSC cocktails are of interest to improve the radiopurity of LSC cocktails. The possibility to reduce potassium contaminations in LSC cocktails by one order of magnitude would result in extremely low-background LSC cocktails that would benefit a wide range of fields, including fundamental science, environmental monitoring, non-proliferation and national security applications.
References
Terlikowska T, Hainos D, Cassette P, Radoszewski T (2000) Application of \(\alpha\)/\(\beta\) discrimination in liquid scintillation counting for the purity control of 99mtc medical solutions. Appl Radiat Isot 52(3):627–632
Hou X (2018) Liquid scintillation counting for determination of radionuclides in environmental and nuclear application. J Radioanal Nucl Chem 318(3):1597–1628
Boireau G, Bouvet L, Collin A, Coulloux G, Cribier M, Deschamp H, Durand V, Fechner M, Fischer V, Gaffiot J (2016) Online monitoring of the Osiris reactor with the Nucifer neutrino detector. Phys Rev D 93(11):112006
Bellini G, Benziger J, Bonetti S, Avanzini MB, Caccianiga B, Cadonati L, Calaprice F, Carraro C, Chavarria A, Chepurnov A (2010) Measurement of the solar b 8 neutrino rate with a liquid scintillator target and 3 mev energy threshold in the borexino detector. Phys Rev D 82(3):033006
An F, Bai J, Balantekin A, Band H, Beavis D, Beriguete W, Bishai M, Blyth S, Brown R, Butorov I (2016) The detector system of the Daya bay reactor neutrino experiment. Nucl Instrum Methods Phys Res, Sect A 811:133–161
Andringa S, Arushanova E, Asahi S, Askins M, Auty D, Back A, Barnard Z, Barros N, Beier E, Bialek A et al (2016) Current status and future prospects of the sno+ experiment. Adv High Energy Phys 2016
Abe S, Ebihara T, Enomoto S, Furuno K, Gando Y, Ichimura K, Ikeda H, Inoue K, Kibe Y, Kishimoto Y (2008) Precision measurement of neutrino oscillation parameters with Kamland. Phys Rev Lett 100(22):221803
Buck C, Yeh M (2016) Metal-loaded organic scintillators for neutrino physics. J Phys G Nucl Part Phys 43(9):093001
Hans S, Cumming J, Rosero R, Perez RD, Reyes CC, Gokhale S, Yeh M (2020) Light yield quenching and quenching remediation in liquid scintillator detectors. J Instrum 15(12):12020
Yeh M, Hans S, Beriguete W, Rosero R, Hu L, Hahn R, Diwan M, Jaffe D, Kettell S, Littenberg L (2011) A new water-based liquid scintillator and potential applications. Nucl Instrum Methods Phys Res, Sect A 660(1):51–56
Verrezen F, Loots H, Hurtgen C (2008) A performance comparison of nine selected liquid scintillation cocktails. Appl Radiat Isot 66(6–7):1038–1042
Medeiros RB, Godinho RO, Mattos M (2003) Comparison of the efficacy of biodegradable and non-biodegradable scintillation liquids on the counting of tritium-and [14c]-labeled compounds. Braz J Med Biol Res 36:1733–1739
Benziger JB, Johnson M, Calaprice F, Chen M, Darnton N, Loeser R, Vogelaar R (1998) A scintillator purification system for a large scale solar neutrino experiment. Nucl Instrum Methods Phys Res, Sect A 417(2–3):278–296
Ford R, Chen M, Chkvorets O, Hallman D, Vázquez-Jáuregui E (2011) Sno+ scintillator purification and assay. In: AIP Conference Proceedings, vol. 1338, pp. 183–194 American Institute of Physics
Alimonti G, Angloher G, Arpesella C, Balata M, Bellini G, Benziger J, Bonetti S, Cadonati L, Calaprice F, Cecchet G (1998) Measurement of the 14c abundance in a low-background liquid scintillator. Phys Lett B 422(1–4):349–358
NCRP (1986) Radiation exposure of the US population from consumer products and miscellaneous sources. NCRP Report No. 95,
di Vacri ML, Arnquist IJ, Scorza S, Hoppe EW, Hall J (2021) Direct method for the quantitative analysis of surface contamination on ultra-low background materials from exposure to dust. Nucl Instrum Methods Phys Res, Sect A 994:165051
Barabanov I, Bezrukov L, Veresnikova A, Gurentsov V, Morgalyuk V, Novikova GY, Yanovich E (2016) A procedure for removing uranium, thorium, and potassium-40 microimpurities from a liquid organic scintillator based on linear alkylbenzene. Radiochemistry 58(1):52–58
Barabanov I, Morgalyuk V, Novikova GY, Yanovich E (2016) Efficiency of different methods for removing u, th, and k from a liquid scintillator. Radiochemistry 58(6):625–630
Hartnell J, the SNO+ collaboration (2012) Neutrinoless double beta decay with sno+. J Phys Conf Ser 375:042015
Erchinger JL, Orrell JL, Aalseth CE, Bernacki BE, Douglas M, Fuller ES, Keillor ME, Marianno CM, Morley SM, Mullen CA (2017) Background characterization of an ultra-low background liquid scintillation counter. Appl Radiat Isot 126:168–170
Aalseth CE, Bonicalzi R, Cantaloub MG, Day AR, Erikson LE, Fast J, Forrester JB, Fuller ES, Glasgow BD, Greenwood LR (2012) A shallow underground laboratory for low-background radiation measurements and materials development. Rev Sci Instrum 83(11):113503
Aalseth CE, Day AR, Fuller ES, Hoppe EW, Keillor ME, LeFerriere B, Mace EK, Merriman J, Myers AW, Overman CT (2013) A new shallow underground gas-proportional counting lab-first results and ar-37 sensitivity. Appl Radiat Isot 81:151–155
Seifert A, Aalseth CE, Day AR, Fuller ES, Hoppe EW, Keillor ME, Mace EK, Overman CT, Warren GA (2013) The design, construction, and initial characterization of an ultra-low-background gas-proportional counting system. J Radioanal Nucl Chem 296:915–921. https://doi.org/10.1007/s10967-012-2059-5
Erchinger JL, Aalseth CE, Bernacki BE, Douglas M, Fuller ES, Keillor ME, Morley SM, Mullen CA, Orrell JL, Panisko ME, Warren GA, Williams RO, Wright ME (2015) Development of a low background liquid scintillation counter for a shallow underground laboratory. Appl Radiat Isot 105:209–218. https://doi.org/10.1016/j.apradiso.2015.08.027
Bernacki BE, Douglas M, Erchinger JL, Fuller ES, Keillor ME, Morley SM, Mullen CA, Orrell JL, Panisko ME, Warren GA (2015) Optical design considerations for efficient light collection from liquid scintillation counters. Appl Opt 54(9):2413–2423
Leonard D, Grinberg P, Weber P, Baussan E, Djurcic Z, Keefer G, Piepke A, Pocar A, Vuilleumier J-L, Vuilleumier J-M (2008) Systematic study of trace radioactive impurities in candidate construction materials for exo-200. Nucl Instrum Methods Phys Res, Sect A 591(3):490–509
Aprile E, Arisaka K, Arneodo F, Askin A, Baudis L, Behrens A, Bokeloh K, Brown E, Cardoso J, Choi B (2011) Material screening and selection for xenon100. Astropart Phys 35(2):43–49
Abgrall N, Arnquist IJ, Avignone Iii F, Back HO, Barabash AS, Bertrand F, Boswell M, Bradley A, Brudanin V, Busch M (2016) The majorana demonstrator radioassay program. Nucl Instrum Methods Phys Res, Sect A 828:22–36
Wang X, Chen X, Fu C, Ji X, Liu X, Mao Y, Wang H, Wang S, Xie P, Zhang T (2016) Material screening with HPGe counting station for PandaX experiment. J Instrum 11(12):12002
Orrell JL, Aalseth CE, Arnquist IJ, Eggemeyer TA, Glasgow BD, Hoppe EW, Keillor ME, Morley SM, Myers AW, Overman CT (2016) Assay methods for 238 u, 232 th, and 210 pb in lead and calibration of 210 bi bremsstrahlung emission from lead. J Radioanal Nucl Chem 309(3):1271–1281
Edelweiss Collaboration. http://radiopurity.in2p3.fr/search.php?Material=Silver
Winn W, Bowman W, Boni A (1988) Ultra-clean underground counting facility for low-level environmental samples. Sci Total Environ 69:107–144
Acknowledgements
Pacific Northwest National Laboratory (PNNL) is operated by Battelle for the United States Department of Energy (DOE) under Contract no. DE-AC05-76RL01830. This work was funded by National Nuclear Security Administration (NNSA) Office of Defense Nuclear Nonproliferation Research and Development. The authors would like to thank Abdul Ibrahim at National Diagnostics, Shaun Smyth, James Thomson, and Vikki Binns from Meridian Biotechnologies, and Betsy Moran from Perkin Elmer for providing samples, and for useful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
di Vacri, M.L., Arnquist, I.J., Back, H.O. et al. Identification of background limitations to ultra-sensitive LSC counting through ICP-MS assay of LSC cocktails. J Radioanal Nucl Chem 331, 5597–5604 (2022). https://doi.org/10.1007/s10967-022-08591-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08591-9