Abstract
Intrinsic \(^{40}\)K radioactive backgrounds from impurities of natural K in liquid scintillation cocktails have previously been demonstrated to limit their use in ultra-sensitive applications. This work explores two methodologies in parallel for the reduction of \(^{40}\)K backgrounds in the cocktails, and lays the groundwork for use in ultra-sensitive applications. In one method, alternative low-K liquid scintillation matrix constituents were identified and in the other, a simple purification method for single components and finished cocktails was developed. Both methods were verified via ICP-MS analysis. Liquid scintillation counting of selected purified cocktails demonstrated background reduction, improved stability, and enhanced performance. The best performing purified cocktail was also counted on a custom-built ultra-low background liquid scintillation counter, with results below the detector background.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liquid scintillation counting (LSC) is a standard technique for radiation detection commonly used for \(\alpha \)/\(\beta \) detection. The technique is utilized in a wide variety of fields such as medical applications [1], environmental processes [2, 3], and non-proliferation monitoring [4, 5]. The sensitivity of LSC detection can be limited by radioactive backgrounds from various sources (e.g., intrinsic contamination in detector materials, cosmic radiation), which can conceal the signature of interest. Additionally, radioactive impurities in LSC cocktails can also contribute to the radioactive background of the detector [6, 7]. Typically, measurable concentrations for naturally occurring radionuclides such as \(^{40}\)K, \(^{232}\)Th, and \(^{238}\)U (and their progeny) are of significant concern for ultra-sensitive detection applications whereas the presence of these radionuclides at low concentrations is typically less of a concern for commercial detectors.
To achieve the utmost sensitivity, radioactive backgrounds are mitigated through a strict selection of radiopure materials for detector construction. Additionally, shielding from cosmic radiation can be obtained by constructing and operating detectors underground [8, 9]. Using this mindset, the Ultra-Low Background Liquid Scintillation Counter (ULB-LSC) at Pacific Northwest National Laboratory (PNNL) was designed and built using radiopure materials in the laboratory’s Shallow Underground Laboratory (SUL) [6, 10, 11]. The SUL provides approximately 30 m water-equivalent cosmic-ray background reduction [11]. The ULB-LSC was designed to provide a 30× background reduction compared to traditional high-sensitivity commercial LSC detectors, and is described in detail in previous work [10]. The expected ULB-LSC detector background is on the order of tens of counts per day (cpd) [6].
Reduction in detector backgrounds represent a notable increase in signal sensitivity, revealing intrinsic radioactive impurities in LSC cocktails. In previous work [7], measurable impurities from natural potassium (\(^{nat}\)K) were identified in commercial LSC cocktails through inductively coupled plasma mass spectrometry (ICP-MS) analysis, and confirmed by high purity germanium counting. Backgrounds from potassium impurities, specifically from radioactive isotope \(^{40}\)K (0.01% natural isotopic abundance), were 10\(^2\)–10\(^3\) cpd for 10 mL sample volumes [7], which is orders of magnitude above the expected ULB-LSC background levels [6]. These results represent a concerning limitation for ultra-low background applications.
LSC cocktails are generally comprised of a solvent base, a primary fluorescent agent (fluor), and a secondary wavelength shifter. An emulsifier, typically a surfactant, is also added (ca. 20–30\(\%\)) to allow analysis of aqueous samples, as non-polar neat organic scintillators are immiscible with aqueous solutions. A further investigation of potassium impurities in individual cocktail constituents via ICP-MS identified the surfactant component as the dominant vector for potassium impurities in the cocktails [7]. This is likely because many surfactants, particularly nonylphenol ethoxylates (NPEs) which have traditionally been used as cocktail surfactants, are synthesized using a potassium-based catalyst [12].
In this work, we have explored developing LSC cocktails with reduced potassium backgrounds targeting ultra-sensitive LSC liquid scintillation applications. We have followed two distinct approaches: (1) start clean, stay clean, in which we investigate reduced-potassium or potassium-free manufacturing of scintillation cocktails, and (2) development of a simple, straight-forward purification process for commercial off-the-shelf (COTS) and custom-made scintillation cocktails. While the aim of the study was to lower backgrounds for ultra-sensitive applications, the cocktails subjected to the developed purification method were also tested in a COTS LSC detector to observe any impacts on a commercial system.
Potassium reduction approaches
Start clean, stay clean
Drawing from concepts typically employed in low-background detector development and ultra-sensitive applications, the start clean, stay clean approach consists of selecting radiopure starting materials and mitigating contamination at each step of the manufacturing process to minimize radioactive backgrounds. By working with manufacturers to create radiopure materials, this approach can be extremely effective for lowering radioactive backgrounds [13, 14]. This method was employed during the design and construction of the ULB-LSC to minimize inherent radioactive backgrounds present in construction materials [6]. The same method can be applied to the manufacturing of LSC cocktails. Following previous work [7], we have investigated potential alternative low-K surfactants with a start clean, stay clean approach towards the manufacturing of low background LSC cocktails [7]. Surfactants with \(^{nat}\)K content one to two orders of magnitude lower compared to those previously reported [7] were identified via ICP-MS analysis. Results are shown in Fig. 1.
Purification
An alternative to the start clean, stay clean method is to purify materials of intrinsic radioactive contamination after manufacturing. Commonly used methods for analyte specific purification processes include procedures such as ion chromatography, liquid-liquid extraction, and precipitation. We have developed a method of purification that is less time-consuming and laborious than the aforementioned techniques, the details of which are patent pending [15]. Several commercially-available LSC cocktails from different manufacturers, as well as a custom cocktail developed at Brookhaven National Laboratory (BNL), went through our purification process. The efficacy of the purification method was verified through ICP-MS analysis of the as-received (referred to henceforth as baseline) and purified cocktails. In order to determine potential performance effects (i.e. scintillation efficiency), baseline and purified samples were spiked with known quantities of radioactive standards (\(^3\)H, \(^{14}\)C, \(^{90}\)Sr) and evaluated using a commercial LSC detector (PerkinElmer Quantulus 1220). Results from these measurements are reported in "Commercial LSC" section. Lastly, to investigate the impact of this method for ultra-sensitive measurements, we measured the backgrounds for a selected purified cocktail on PNNL’s ULB-LSC ("ULB-LSC" section).
Trace K analysis methodology
ICP-MS
Ten surfactants were analyzed as part of the start clean, stay clean investigation, since surfactants are the dominant vector for elevated potassium backgrounds. Six surfactants from the low-K, NPE-free Novel line were received from Sasol (Sandton, South Africa), and four surfactants were received from BNL. Further details about the BNL surfactants were not provided to comply with BNL Intellectual Property. For the development and validation of the purification technique, six surfactant constituents from two commercial companies, National Diagnostics and Meridian, were selected. Since cocktail recipes tend to be proprietary, no further information was provided for these six surfactants. Additionally, three commercial cocktails (PerkinElmer Ultima Gold uLLT, National Diagnostics Ecoscint Ultra, and Meridian Gold Star LT\(^{2}\)) and one custom cocktail (Brookhaven National Laboratory) were used to test the purification technique.
Assay methods followed those as described in detail in di Vacri et al. 2022 [7]. Ultra-low background perfluoroalkoxy alkane (PFA) screw cap vials from Savillex (Eden Prairie, MN) were used for preparation of reagent solutions and sample dilutions for ICP-MS analyses. Polyethylene (PE) LSC vials (PerkinElmer, Waltham, MA) were used for sub-sampling and first level dilutions of the samples. PFA vials (20 mL; PerkinElmer) were also used for sample counting in the ULB-LSC.
Optima grade nitric and hydrochloric acids (Fisher Scientific, Pittsburg, PA) and 18.2 M\(\Omega \cdot \)cm deionized water from a MilliQ system (Merk Millipore GmbH, Burlington, MA) were used for preliminary labware cleaning and preparation of reagent solutions. All labware underwent preliminary cleaning and validation before use [7].
A class 10,000 cleanroom and a laminar flow hood providing a class 10 environment at PNNL were used for sample preparation and analyses. Measurements were performed using an Agilent 8900 triple quadrupole ICP-MS (Agilent Technologies, Santa Clara, CA), equipped with an integrated autosampler, a microflow PFA nebulizer and a quartz double pass spray chamber. Potassium determinations were performed in cool plasma with NH\(_3\) in MS/MS mode. Plasma, ion optics, and mass analyzer parameters were optimized based on the instrumental response from a prepared 1 ng\(\cdot \)g\(^{-1}\) K standard solution. The acquisition method included three replicates and ten sweeps per measurement. Acquisition times were set based on the expected signal, in order to maximize the instrumental precision by improving counting statistics. Quantitation of \(^{nat}\)K was performed through an external calibration curve, using potassium standards with natural isotopic composition (Inorganic Ventures, Christiansburg, VA). The signal at m/z of 39, pertaining to the most abundant potassium isotope (\(^{39}\)K) was used as the signal for quantitation, and was cross-checked with m/z 41 (\(^{41}\)K) for isotopic anomalies. All samples measured were at natural isotopic abundance levels for K. Samples were diluted in 2\(\%\) nitric acid solution and were prepared and analyzed in triplicate. Three process blanks were prepared for each unique set of measurements, and signals from samples were process blank subtracted. It is worth pointing out that ICP-MS does not detect radiation from decays, it measures ions. Therefore, the ICP-MS results reported here are in concentrations (e.g., ppm or \(\mu \)g\(\cdot \)g\(^{-1}\) of sample), but when required, corresponding activities (e.g., mBq\(\cdot \)kg\(^{-1}\)) were calculated based on the specific activity and natural isotopic abundance \(^{40}\)K. For reference, 1 \(\mu \)g\(\cdot \)g\(^{-1}\) \(^{nat}\)K corresponds to 30.5 mBq\(\cdot \)kg\(^{-1}\) from \(^{40}\)K.
Commercial LSC
A PerkinElmer Quantulus 1220 liquid scintillation counter was used to measure baseline and purified samples (PerkinElmer Ultima Gold uLLT, National Diagnostics Ecoscint Ultra, and Meridian Gold Star LT\(^{2}\)) at Savannah River National Laboratory (SRNL). Samples were retained in 20 mL low-diffusion anti-static PE counting vials, were light- and temperature-adapted for a minimum of 12 h prior to initial sampling, and were stored in a dark, temperature-controlled chamber until completion of sampling. Spectral data were acquired across the full energy spectral region (channels 1–1024) with the instrument held at a fixed operating temperature of 18 \(^{\circ }\)C. Data were collected sequentially in quintuplicate for a duration of 12 h for each measurement.
K mitigation in LSC cocktails
ICP-MS assay results
Start clean, stay clean: alternative surfactants
Potassium concentrations for alternative, low-K surfactants from Sasol and BNL are reported in Fig. 1. The data are represented as the average and standard deviation of three replicates, unless otherwise noted, and are compared to the average content of potassium in surfactants used in commercial LSC cocktails [7]. In nine of the ten samples, potassium content is well below the average concentration in the other COTS surfactants, in some cases by two orders of magnitude. This result indicates that these surfactants are potential radiopure starting material for manufacturing low-background LSC cocktails within a start clean, stay clean approach. We have not tested the performance of these surfactants when incorporated in LSC cocktails, as that investigation was beyond the scope of this study. However, other workers [16, 17] have shown the efficacy of other NPE-free surfactants in LSC cocktails. The commercially available low-K, NPE-free surfactants tested in this study may be appropriate alternatives to high-potassium surfactants in commercial or custom LSC cocktails.
Potassium concentrations (\(\mu \)g\(\cdot \)g\(^{-1}\)) in a series of low-K surfactants. Samples 1–6 (dark blue) are from the Novel line by Sasol and samples 7–10 (light blue) are from Brookhaven National Laboratory). All reported values are upper limits, aside from samples 8 and 9, denoted by an asterisk. The average concentration of COTS surfactants typically used in liquid scintillation cocktails is denoted by the red-dashed line. (Color figure online)
Purification
Surfactants
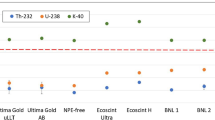
Selected previously analyzed surfactants [7] were used to test the efficacy of the purification method developed within this work. Figure 2 shows \(^{nat}\)K concentrations measured in the surfactants via ICP-MS before and after application of the purification method. Our method reduced \(^{nat}\)K impurities by a factor of four, on average, for all purified surfactants. Aside from National Diagnostics surfactant sample 6, for which the method was not as effective, all other surfactants had an average \(^{nat}\)K reduction of a factor of ten. Before purification, surfactants had \(^{nat}\)K impurities ca. 5 times higher than commercial as-purchased LSC cocktails. Assuming no change in efficacy, using a purified surfactant in a commercial or custom LSC cocktail could reduce \(^{nat}\)K impurities in the final cocktail by an order of magnitude.
LSC cocktails
Four LSC cocktails (three commercial, one custom) were purified using the same method used for the surfactants. Results are shown in Fig. 3. All cocktails showed reduction of \(^{nat}\)K concentrations after purification, where values ranged from a factor of 7 to a factor of 38. The purification method proved especially effective for the PerkinElmer Ultima Gold uLLT and National Diagnostics Ecoscint Ultra cocktails, with reduction factors of 38× and 35×, respectively. Meridian GoldStar LT\(^{2}\) and BNL House cocktail’s \(^{nat}\)K concentrations were reduced by factors of 7× and 9×, respectively. Although there was variability in the purification efficiencies amongst the various cocktails, the purification method reduced \(^{nat}\)K in all the cocktails by roughly one order of magnitude. In addition, the purification method lowered \(^{nat}\)K concentrations in two commercial cocktails with the highest baseline contamination to near or below that of Ultima Gold uLLT, which has the lowest baseline contamination for \(^{nat}\)K for the commercial cocktails measured.
Impact
Commercial LSC
Baseline and purified cocktails were evaluated for background contributions by LSC to demonstrate the effects of the purification method on commercial cocktails. Spectral data were acquired across the full energy region of interest (ROI) including \(^{40}\)K contributions, if present (K-ROI; channels 500–1000), and are shown in Table 1. For consistency and comparison with ULB-LSC backgrounds, results are reported as cpd for each sample. In addition, a brief scoping study to demonstrate LSC performance capabilities for purified cocktails was conducted. A 5.5 mL aliquot from each of the six primary samples (baseline and purified versions of the three commercial cocktails) was transferred to four pre-weighed 6 mL anti-static Pico Prias (PerkinElmer) vials. One vial was reserved for background measurements due to the vial geometry changes and the other three were each spiked with one of three calibrated NIST traceable isotope solutions (Eckert & Ziegler Isotope Products, Berlin, Germany). Selected to cover a range of energies and matrices, the calibrated solutions included a low-energy beta in water (\(^{3}\)H), a medium-energy beta in base (\(^{14}\)C in 0.1 M NaOH), and a high-energy beta in acid (\(^{90}\)Sr in 0.1 M HCl). Each respective Pico vial with transferred cocktail, baseline or purified, was spiked with 100 \(\mu \)L of calibrated solution for a decay-corrected activity of 16 decays per minute total in solution. LSC measurements were analyzed for the optimal ROI specific to each isotope: channels 50–320 for \(^{3}\)H, channels 50–650 for \(^{14}\)C, and channels 50–920 for \(^{90}\)Sr/\(^{90}\)Y, as well as the full energy range (channels 1–1024).
Results from the measured subaliquots were used to calculate efficiencies and estimate the minimum detectable activity (MDA) and figure of merit (FOM) for each cocktail. The MDAs were used to evaluate background measurements as recognized values consistent and quantifiable for low-level counting applications [10], and are shown in Table 2. MDA calculations were made using the following equation [18]:
where, for a specified geometry, \(L_D\) represents the detection limit, \(C_b\) is the background count rate in counts per day, \(T_b\) is the background count time in days, \(\epsilon \) is the counting efficiency for the specified geometry and matrix, \(V_s\) is the sample mass in grams, and \(T_s\) is the sample count time in minutes. The FOM parameter was used to comparatively assess the sensitivity, or signal-to-noise, for the spiked cocktail solutions counted by LSC [19,20,21,22]. Higher fidelity measurements would be indicated by a larger FOM value, as it relates counting efficiency performance and background count rates. FOM values were estimated using the equation below, and improvements from purification as measured by LSC are shown in Table 3:
where \(\epsilon \) is the counting efficiency for the specified geometry and matrix and b is the background count rate in counts per day.
Initial performance testing on a commercial LSC detector for the purification of these commercial cocktails showed consistent improvements with slightly lower MDAs and higher FOMs than baseline forms. In addition, observed counting statistics between repeat measurements were consistent.
An investigation of the stability of spiked cocktails was performed with repeat measurements up to 180 days post-sample preparation. Using the FOM values shown in Table 3 for comparison, the samples demonstrated consistently high stability for \(^{3}\)H in water and \(^{90}\)Sr in dilute acid for both baseline and purified samples (<10% reduction in FOM). However, \(^{14}\)C in dilute base did not maintain stability. After 180 days, the FOM had reduced by 96% for baseline samples and between 24–65% for purified samples, with Ultima Gold uLLT demonstrating the least reduction in FOM. While \(^{14}\)C in base did not show the same stability as the other two spikes, the purified sample was significantly more stable over time.
ULB-LSC
To understand the full extent of the impact of the purification method on ultra-sensitive LSC measurements, the cocktail that demonstrated the best \(^{nat}\)K-reduction was tested on the ULB-LSC. An ultra-low background PFA vial was filled with 20 mL of baseline and one with purified PerkinElmer Ultima Gold uLLT and counted for one week. The instrument background was determined by measuring a cylindrical piece of polyvinyl toluene (PVT), which is a known radiopure scintillator. This provided a measurement of the intrinsic background of the detector itself to which the baseline and purified could be compared. The resultant count rate for the PVT was 80 cpd (K ROI), 205 cpd (full spectrum), which is the measured background for the instrument. Figure 4 shows the spectra from the uLLT contributions (baseline and purified) over the entire channel range for the instrument compared to the intrinsic instrument background. The difference between the baseline cocktail (in black) and the purified cocktail (in orange) indicate an observed reduction from the purification technique of roughly 4x. While this is not in agreement with the ICP-MS results, it is likely because the purified sample is below the ULB-LSC’s background count rate as demonstrated by the statistical agreement with the PVT spectra (in blue). Therefore, it was concluded that the purified Ultima Gold uLLT cocktail would yield the lowest possible backgrounds for sample counting in the ULB-LSC.
Conclusions
Intrinsic radioactive impurities in commercial LSC cocktails, namely from long-lived radionuclide \(^{40}\)K, have presented a limitation for ultra-low background LSC detection [7]. Two techniques have been explored to mitigate this limitation: start clean, stay clean, which provided a potential solution to high \(^{nat}\)K concentrations by replacing a problematic constituent (surfactants) with low-K alternatives; and purification, which removed \(^{nat}\)K contamination in the finished LSC cocktail by at least an order of magnitude. Through the development of a fast and simplistic purification technique, this study has shown that reducing the levels of potassium in commercial LSC cocktails significantly improves performance on a commercial LSC detector by lower minimum detectable activity and higher detection efficiencies and figure of merit values. In addition, the purification method lowered a commercial cocktail’s \(^{40}\)K below the background count rate of a custom-made ultra-low background detector, laying the groundwork for ultra-sensitive LSC detectors and applications.
References
Terlikowska T, Hainos D, Cassette P, Radoszewski T (2000) Application of \(\alpha \)/\(\beta \) discrimination in liquid scintillation counting for the purity control of 99mTc medical solutions. Appl Radiat Isot 52(3):627–632. https://doi.org/10.1016/S0969-8043(99)00221-3
Kim HR, Choi GS, Park SY, Lee CW, Han MH (2010) The radioactivity of 3H in metals by a high temperature furnace and a liquid scintillation counter. In: International Conference on Radioactive Waste Management and Environmental Remediation, vol 54525, pp 491–496. https://doi.org/10.1115/ICEM2010-40181
Lauria D, Carvalho L, Conti C (2006) Comparison of different methods for 210Pb determination in environmental samples. Adv Liq Scintillation Spectrom LSC 2005:211–216
Hou X (2018) Liquid scintillation counting for determination of radionuclides in environmental and nuclear application. J Radioanal Nucl Chem 318(3):1597–1628. https://doi.org/10.1007/s10967-018-6258-6
Boireau G, Bouvet L, Collin A, Coulloux G, Cribier M, Deschamp H, Durand V, Fechner M, Fischer V, Gaffiot J (2016) Online monitoring of the Osiris reactor with the Nucifer neutrino detector. Phys Rev D 93(11):112006. https://doi.org/10.1103/PhysRevD.93.112006
Erchinger JL, Orrell JL, Aalseth CE, Bernacki BE, Douglas M, Fuller ES, Keillor ME, Marianno CM, Morley SM, Mullen CA (2017) Background characterization of an ultra-low background liquid scintillation counter. Appl Radiat Isot 126:168–170. https://doi.org/10.1016/j.apradiso.2017.01.032
di Vacri M, Back H, Bliss M, Bronikowski M, Edwards E, Hackett B, Hoppe E, Lyons S, Rocco N (2022) Identification of background limitations to ultra-sensitive LSC counting through ICP-MS assay of LSC cocktails. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-022-08591-9
Aalseth CE, Day AR, Fuller ES, Hoppe EW, Keillor ME, LeFerriere B, Mace EK, Merriman J, Myers AW, Overman CT (2013) A new shallow underground gas-proportional counting lab-first results and Ar-37 sensitivity. Appl Radiat Isot 81:151–155. https://doi.org/10.1016/j.apradiso.2013.03.050
Seifert A, Aalseth CE, Day AR, Fuller ES, Hoppe EW, Keillor ME, Mace EK, Overman CT, Warren GA (2013) The design, construction, and initial characterization of an ultra-low-background gas-proportional counting system. J Radioanal Nucl Chem 296:915–921. https://doi.org/10.1007/s10967-012-2059-5
Erchinger JL, Aalseth CE, Bernacki BE, Douglas M, Fuller ES, Keillor ME, Morley SM, Mullen CA, Orrell JL, Panisko ME, Warren GA, Williams RO, Wright ME (2015) Development of a low background liquid scintillation counter for a shallow underground laboratory. Appl Radiat Isot 105:209–218. https://doi.org/10.1016/j.apradiso.2015.08.027
Aalseth CE, Bonicalzi R, Cantaloub MG, Day AR, Erikson LE, Fast J, Forrester JB, Fuller ES, Glasgow BD, Greenwood LR (2012) A shallow underground laboratory for low-background radiation measurements and materials development. Rev Sci Instrum 83(11):113503. https://doi.org/10.1063/1.4761923
Dow: Product safety assessment: Dow nonylphenol ethoxylate surfactants. Technical report, The Dow Chemical Company (2010)
Arnquist IJ, Beck C, di Vacri ML, Harouaka K, Saldanha R (2020) Ultra-low radioactivity Kapton and copper-Kapton laminates. Nucl Instrum Methods Phys Res, Sect A 959:163573. https://doi.org/10.1016/j.nima.2020.163573
Arnquist IJ, di Vacri ML, Rocco N, Saldanha R, Schlieder T, Patel R, Patil J, Perez M, Uka H (2023) Ultra-low radioactivity flexible printed cables. arXiv preprint arXiv:2303.10862. https://doi.org/10.48550/arXiv.2303.10862
Arnquist IJ, di Vacri ML, Rocco N, French A. Method for purification and removal of potassium from nonpolar and surfactant solutions and mixtures. PCT/US2022/045290; WO/2023/055975. https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2023055975 &_cid=P12-LKTUEM-60611-1
Vasile M, Loots H, Vercammen L, Bruggeman M, Verrezen F (2022) A study for the selection of NPE-free cocktails for LSC routine measurements. J Radioanal Nucl Chem 331(8):3349–3357. https://doi.org/10.1007/s10967-022-08405-y
Varlam C, Vagner I, Faurescu D (2019) Performance of nonylphenol-ethoxylates-free liquid scintillation cocktail for tritium determination in aqueous samples. J Radioanal Nucl Chem 322(2):585–595. https://doi.org/10.1007/s10967-019-06702-7
Currie LA (1968) Limits for qualitative detection and quantitative determination. Application to radiochemistry. Anal Chem 40(3):586–593. https://doi.org/10.1021/ac60259a007
Jakonić I, Todorović N, Nikolov J, Bronić IK, Tenjović B, Vesković M (2014) Optimization of low-level LS counter Quantulus 1220 for tritium determination in water samples. Radiat Phys Chem 98:69–76. https://doi.org/10.1016/j.radphyschem.2014.01.012
Verrezen F, Loots H, Hurtgen C (2008) A performance comparison of nine selected liquid scintillation cocktails. Appl Radiat Isot 66(6–7):1038–1042. https://doi.org/10.1016/j.apradiso.2008.02.050
Zhilin C, Shixiong X, Heyi W, Yinhang Z (2010) The effect of vial type and cocktail quantity on tritium measurement in LSC. Appl Radiat Isot 68(9):1855–1858. https://doi.org/10.1016/j.apradiso.2010.04.015
Sanchez-Cabeza J, Pujol JM, Luis Leon JM, Vidal-Quadras A, Mitchell P (1993) Optimization and calibration of a low-background liquid scintillation counter for the simultaneous determination of alpha and beta emitters in aqueous samples. Radiocarbon 3:43–50
Acknowledgements
Pacific Northwest National Laboratory (PNNL) is operated by Battelle for the United States Department of Energy (DOE) under Contract no. DE-AC05-76RL01830. This work was funded by National Nuclear Security Administration (NNSA) Office of Defense Nuclear Nonproliferation Research and Development. The authors would like to thank Abdul Ibrahim at National Diagnostics, Shaun Smyth, James Thomson, and Vikki Binns from Meridian Biotechnologies, and Betsy Moran from PerkinElmer for providing samples, and for useful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rocco, N.D., Arnquist, I.J., Back, H.O. et al. Impact of lowering potassium contamination in liquid scintillation cocktails for ultra-sensitive radiation detection. J Radioanal Nucl Chem 332, 4223–4229 (2023). https://doi.org/10.1007/s10967-023-09105-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09105-x