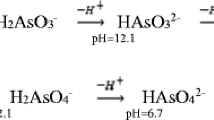Abstract
The spent biological activated carbon (SBAC) as solid waste is used to study the removal of radioactive Sr2+ in water. The results show that SBAC adsorbs Sr2+ reaching equilibrium within 3 min and the adsorption is an exothermic reaction. The removal rate can reach more than 85%, desorption rate is less than 6.16%, and it can also achieve 40% removal in river water. The three-round regeneration efficiencies are all ~ 100%. The adsorption process is without secondary pollution. SBAC has good potential for the removal of radioactive Sr2+ in water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the development of the nuclear industry, radioactive waste is produced in large quantities, and the discharge of radioactive waste is an important source of radionuclides entering the environment. Among them, radioactive strontium (90Sr) has a half-life of 28.8 years and a large share of radioactivity, which is the main nuclides in radioactive water. It is a kind of osteogenic β-radiation source [1, 2]. The biochemical properties similar to calcium will make it participate in the metabolic process. It is difficult to excrete after ingestion, leading to white blood cells, red blood cells and platelets are significantly reduced, regenerative disorders occur, and even lead to leukemia or osteosarcoma [3, 4]. Therefore, the removal of 90Sr has been receiving widespread attention.
To date, techniques for removing radioactive strontium (Sr2+) from aqueous solutions include adsorption, chemical precipitation, membrane separation, and solvent extraction [5,6,7,8,9] etc. Among them, the chemical precipitation method may introduce a large number of salts, and some extractants in the extraction method are highly toxic and the membrane separation method has a low yield [5]. Therefore, the technical choice of treating radioactive containing Sr2+ is increasingly inclined to adsorption. Many researchers have tested the removal of Sr2+ by a variety of organic and inorganic adsorbents, including diatomaceous earth [10], goethite [11], hematite [12], bentonite [13], kaolinite [14], montmorillonite [15], clay minerals [16], pecan shells [17], zeolites [18] and activated carbon (AC), as well as some new adsorbents [19] etc. Although, due to high surface area, porous structure and functional group, AC has been the most popular and widely used adsorbent in water treatment technology all over the world and is used to remove a broad spectrum of impurities from water [20], there are few studies on the adsorption of Sr2+ by AC, and the effect is not satisfactory. Shawabkeh et al. (2002) showed that the removal rate of AC to Sr2+ was 56.3% when the AC dosage was 1 g L−1 [17]. The AC used by Chegrouche et al. (2009) had a removal rate of 60% after 8 h of adsorption under optimal conditions [21]. Moloukhia et al. (2016) modified the coconut shell charcoal to adsorb a variety of radioactive elements, and the adsorption of Sr2+ was less than 40% under the condition of 10 g L−1 dosage and 4 h contact time [22]. Studies by Caccin et al. (2013) and Kubota et al. (2013) have even shown that the ACs they used is difficult to remove Sr2+ [23, 24].
As can be seen that the effect of AC on Sr2+ removal is not ideal, but Andersson et al. (2001) pointed out in the research that the granular activated carbon (GAC) used in the BAC process for a certain period of time adsorbed a large amount of calcium, aluminum, and a certain amount of iron, copper, and cadmium [25]. This proves that some changes have taken place in GAC during the BAC process. Will these changes bring new peculiar properties to AC? Dong et al. (2018, 2020) confirmed that the spent biological activated carbon (SBAC) from BAC process can adsorb metal ions lead and cadmium with the maximum removal rate of > 90% [20, 26]. Moreover, SBACs irrespective of using-time presented stable adsorption abilities (> 99%) for Pb2+ (2.0–8.0 mg L−1) with the maximum uptake of 168.07 mg g−1, and their adsorption mechanism for Pb(II) were confirmed, including the dominant ion exchange (H+, Ca2+ etc.) and metal complexation with hydroxyl and carboxyl functional groups [26]. This confirms the ability of SBAC to adsorb metal ions. On this basis, this work use SBAC to study the removal and application of Sr2+ in water. Undoubtedly, this will not only help to find a new low-cost adsorbent for the possible radioactive Sr2+ pollution, but also make the widely used BAC advanced feedwater treatment process safer in case of sudden radioactive contamination, that is, the BAC process has the ability to deal with radioactive strontium pollution.
Using stable isotope of Sr, which has similar chemical property as radioactive strontium (90Sr) [27], the potential of SBAC for the removal of radionuclides Sr2+ were studied in this paper. SBACs with different using-time were sampled from the same drinking water treatment plant with our previous study [20, 26]. The main objectives of this study are: (1) to characterize the adsorption of Sr2+ by SBAC, (2) to study the adsorption properties of Sr2+ from the perspectives of kinetics, isotherms, thermodynamics and other influencing factors, (3) to study the adsorption mechanism, (4) to test the applicability of SBAC to Sr2+ adsorption.
Experimental
The SBAC samples taken from a certain drinking water treat plant (used 5, 6 and 7 years) called SBAC-5, SBAC-6, SBAC-7, were naturally dried. Then the samples were ground to a powder until 95% of the SBAC particles passed through a 325-mesh sieve, and then dried at 80 °C for 3 h in a vacuum drying oven. The Sr2+ water solution was prepared by dissolving strontium chloride hexahydrate (natSr, SrCl2·6H2O, 99%, Tianjin Guangfu Fine Research Institute, China) in pure water (Elix 10, 15.0 MΩ cm at 298 K). Sodium hydroxide (NaOH, AR, Tianjin No. 3 Chemical Reagent Factory, China), Hydrochloric acid, Nitric acid (HCl, AR, HNO3, AR, Tianjin Damao Chemical Reagent Factory, China), Calcium chloride dihydrate, Magnesium chloride dihydrate (CaCl2·2H2O, AR, MgCl2·2H2O, AR, Tianjin KeMiou Chemical Reagent Co., Ltd., China), Cesium chloride (133Cs, CsCl, 99%, Tianjin Guangfu Fine Research Institute, China) were used in this work.
The concentrations of Sr2+ and other metal ions were determined by ICP-OES (iCAP™ 7400, Thermo Electron Corporation, USA). The pH value was measured using a portable multimeter equipped with a pH probe (HQ40D, Hach). Fourier transform infrared (FTIR) spectroscopy was performed on a NEXUS 870 pectrophotometer using the KBr disk method (IRAffinity-1S). X-ray photoelectron spectroscopy (XPS) (Thermo Scientific ESCALAB 250 spectrometer) determined the elemental composition and chemical bonding state of the SBACs sample. All binding energy values were corrected from adventitious hydrocarbon to the C 1s line at 284.8 eV.
According to ASTM D3860-98 (2014) [28] standards, the adsorption experiments were respectively conducted as follows.
The precisely weighed adsorbent (SBAC) was mixed with a certain initial concentration of Sr2+ solution in a centrifuge tube. Considering possible water pollution incidents, drinking water quality requirements and detection limit of ICP-OES, the initial concentration of Sr2+ was set to approximately 5 mg L−1. The centrifuge tube containing the mixture was placed in a thermostatic shaker (HT-2102C, Herrytech) and shaken at a specific temperature. When the adsorption equilibrium was reached, the adsorbent was immediately filtered through 0.45 µm filter to remove the SBAC. The filtrate was acidulated using 1% HNO3 before the concentration of Sr2+ was determined by ICP-OES. All cases were duplicated under the identical conditions and the results recorded as average values.
The amount of Sr2+ adsorbed on SBAC (qe, mg g−1) [29], adsorption efficiency (Ads, %) [19] and distribution coefficient (Kd, mL g−1) [30, 31] were respectively calculated by the following equations:
where Co (mg L−1) and Ce (mg L−1) are the initial and equilibrium concentrations of Sr2+ in aqueous solutions, respectively, m (g) is the mass of the adsorbent, and V (L) is the aqueous volume.
The adsorption isotherm, thermodynamics and kinetics were studied according to the method of Sr2+ batch adsorption experiments.
The kinetic equations, isothermal models and thermodynamic study used to fit the adsorption experiment data are shown in Table 1.
The influence factors on Sr2+ adsorption were investigated according to the batch models including dosage of SBACs (0–8 g L−1), the initial concentration of Sr2+(5–100 mg L−1), the pH of the solution (2.6–11, 0.1 M HNO3 or 0.1 M NaOH was added to the solution), the temperature of adsorption (283–313 K), coexisting cations (dissolving CaCl2·2H2O and MgCl2·2H2O in Sr2+ solution, total concentration of Ca2+ and Mg2+ is in the range of 0–200 mg L−1).
Results and discussion
Adsorption kinetics
To study the kinetics of Sr2+ adsorption, 2.00 g L−1 of the adsorbent was added into 5.78 mg L−1 Sr2+ solution at 298 K and the remaining Sr2+ concentration was tested at various contact times (0, 1, 3, 5, 10, 20, 45, 60 min). The results were shown in Fig. 1a and Table 2. The dosage of the adsorbent was set to 2 g L−1, which is completely sufficient when the initial concentration of Sr2+ is 5.78 mg L−1 as shown at “Adsorption capacity” section.
Figure 1 shows the adsorption kinetic curves of Sr2+ on SBAC-5, SBAC-6 and SBAC-7. It can be seen from Fig. 1a that the concentration of Sr2+ in the solution drops rapidly in the first few minutes of the adsorption process, and Ads of Sr2+ reaches 85% in almost 3 min. As the adsorption time continues to increase, Ads of Sr2+ does not change much, only increases to about 86.6%. This shows that the reaction between Sr2+ and SBAC is instantaneous and exhibits the characteristics of a chemical reaction.
The pseudo first order, the pseudo second order and intraparticle diffusion models were used to fit the kinetics of Sr2+ adsorption, respectively. The results are shown in Fig. 1b–d. It can be seen from Fig. 1 and Table 2 that the adsorption kinetics of Sr2+ by SBAC-5, SBAC-6, and SBAC-7 completely conform to the pseudo second order, and the R2 value of the linear fit all reaches 1. It can be seen from the kinetic analysis that SBAC reaches equilibrium very quickly during the adsorption of Sr2+, and Ads reaches 85%, which is very beneficial for practical applications.
Adsorption isotherms and thermodynamics
The adsorption isotherms and thermodynamic study were tested at 283, 298 and 313 K by changing the initial concentration of Sr2+ (5.0–200 mg L−1). And thermodynamic calculations were performed based on the above results.
Langmuir isotherm, Freundlich isotherm and Dubinin–Radushkevich (D–R) isotherm were respectively used to fit the inter action between SBAC and Sr2+. The fitting results show that the adsorption isotherms of SBAC-5, SBAC-6 and SBAC-7 are more consistent with the Langmuir model, as shown in Fig. 2a–c, the correlation coefficient R2 is greater 0.976, indicating that Sr2+ had monolayer adsorption on the surface of SBAC. As can be obtained from Table 3 the maximum saturated adsorption capacity of SABC-7 is 30.98 mg g−1, followed by SBAC-6, was 30.41 mg g−1, SBAC-5 was 29.33 mg g−1 at room temperature of 298 K. Considering the practical application, the reaction temperature selected in this paper is 298 K (room temperature).
As seen from Fig. 2d that the curve of lnKC versus 1/T of the van’t Hoff equations is a linear equation with a higher regression coefficient, and R2 values of SBACs are 0.9765, 0.8574, and 0.9509, respectively. Therefore, the thermodynamic parameters can be obtained based on the above results, and the calculation results are listed in Table 3. It can be seen from Table 3 that the adsorption capacity qm of SBAC decreases with increasing temperature, indicating that low temperature is more conducive to the adsorption of Sr2+ by SBAC, which is consistent with the logical relationship between the thermodynamic parameter \(\Delta {\text{H}}^{\Theta }\) and a negative value. In addition, the \(\Delta {\text{G}}^{\Theta }\) is negative value, indicating that the adsorption of Sr2+ on SBACs is a spontaneous process. The \(\Delta {\text{H}}^{\Theta }\) is negative, indicating that the adsorption process is exothermic, so the lower temperature is beneficial to adsorption.
Comparison of Sr2+ adsorption with other AC adsorbents
Table 4 shows the comparison of Sr2+ adsorption with other adsorbents. It can be seen from Table 4 that compared with the other AC adsorbents, Ads of SBAC is the highest.
Influence factors on Sr2+ adsorption
Adsorption capacity
Figure 3 shows the effect of SBAC dosage (0–8 g L−1) on the removal of Sr2+. As can be seen from Fig. 3, when the initial concentration of the Sr2+ solution is 5 mg L−1, with the increase of the dosage of SBAC from 0 to 2 g L−1, the Ads of Sr2+ by SBAC with different service years rapidly increased to more than 85%. With the further increase of SBAC dosage from 2 to 8 g L−1, the Ads of Sr2+ increased slowly to 89% and reach equilibrium. Therefore, the dosage of adsorbent in this work was chosen to be 2.0 g L−1.
It can be seen that there is a big breakthrough comparing with Ads of 16% of the virgin AC at dosage of 2 g L−1 and initial concentration of 5 mg L−1 (Fig. 4a). It should be attributed to be the changes of the surface functional groups and the increasing of metal ions absorbed in AC during BAC process (“Adsorption mechanism” section and Supplementary information). The results of FT-IR and XPS show that compared with virgin AC, SBAC has more oxygen-containing functional groups and calcium and magnesium content on its surface (Fig. S4, Tab. S2). It proves that SBAC from the BAC process did have the potential of removing Sr2+ from water.
Then the effects of different initial Sr2+ concentrations (5–100 mg L−1) were investigated, while keeping other conditions unchanged.
It can be seen from Fig. 4 that when the dosage of adsorbent is 2 g L−1, Ads gradually decreased with the increase of the initial concentration due to insufficient SBAC dosages. That is, when the concentration of Sr2+ in the water to be treated is high, it is necessary to increase the dosage of SBAC to achieve the desired adsorption effect.
The affinity of the material for Sr2+ can be described by Kd. Compared to other AC adsorbents, such as, the modified activated carbon (by oxidation using H2O2 and HNO3) [22] showed Kd values of 63.08 ml g−1 at pH = 5.6, activated carbon A-14 [35] showed Kd values of 10 mL g−1. As shown in Fig. 4b, SBAC material had a high affinity for Sr2+, particularly at low Sr2+ concentrations, the values of Kd were higher than 103 mL g−1 within the Co range of 5–15 mg L−1.
Moreover, the desorption experiments of SBAC after adsorption of Sr2+ samples (SBAC-Sr) also prove the strong adsorption capacity of SBAC for Sr2+, which were performed by adding SBAC-Sr (2 g L−1) in pure water (pH ~ 6.1, 298 K) and shaking for 12 h. The desorption rates of Sr2+ are shown in Table 5. It can be seen that SBACs released a very small portion of loaded Sr2+ (4.43%, 6.16%, 3.90%) into water, implying that Sr2+ was firmly bound on SBACs. Therefore, in terms of the desorption after adsorption is concerned, the amount of Sr2+ adsorbed by SBAC is much higher than the amount of desorption.
Based on the above adsorption test and desorption test, it can be concluded that SBAC has a great advantage over virgin AC in ability to adsorb Sr2+. At the same time, according to the desorption test, it can be seen that the adsorption of SBACs on Sr2+ is stable.
Effect of pH on Sr2+ adsorption
Since the pH of the radioactive water may be extremely acidic or alkaline, it is necessary to study the effect of pH on the adsorption of Sr2+. The effect of the initial pH on the removal efficiency of Sr2+ by SBACs is presented in Fig. 5. Six different pH were tested (2.44, 4.14, 8.20, 10.12, 10.95 and 11.70). It can be seen from Fig. 5 that when the pH at about 2.0, Ads of Sr2+ is very low for all SBACs. With the increase of pH from 4.0 to more than 11.0, Ads is significantly increased and increased to 99.9%. This should be attributed to that the isoelectric point of SBAC is less than 4.14 (SBAC-5, SBAC-6, SBAC-7 all less than 2.86, [26]). Under extremely acidic conditions, the surface of SBAC is protonated because of the high concentration of H+, which causes a strong positive charge on the surface of SBAC, thereby preventing the adsorption of Sr2+ by SBAC due to electrostatic repulsion. When the pH of the solution is higher than the isoelectric point of SBAC, the surface of SBAC is negatively charged, which helps SBAC to adsorb positively charged metal ions, such as Sr2+. Therefore, in addition to the strong acid environment, SBAC is negatively charged in the aqueous solution, which provides a strong basic condition for SBAC to adsorb metal ions.
Effect of coexisting ions on Sr2+ adsorption
Since Ca2+ and Mg2+ are widely present in actual waters, coexisting ions solution are prepared using CaCl2·2H2O and MgCl2·2H2O. Ca2+ and Mg2+ with concentrations ranging from 0 to 200 mg L−1 (0 mg L−1, 50 mg L−1, 100 mg L−1, 150 mg L−1, and 200 mg L−1) was added to approximately 5.0 mg L−1 Sr2+ solution. Adsorption experiments of SABC-5, SABC-6 and SABC-7 on Sr2+ were respectively carried out in above coexisting solutions, and the results are shown in Fig. 6.
It can be seen that compared with the absence of coexisting ions, Ads of Sr2+ by SABC-5, SABC-6 and SABC-7 decreased significantly with the increase of Ca2+ and Mg2+ ions, decreasing from 88% to about 30%. It should be attributed to the competition of Sr2+, Ca2+ and Mg2+ for the same adsorption active sites, which verifies that Sr2+ has the similar biochemical properties with Ca2+ [3]. Undoubtedly, if Sr2+ enters the drinking water system, it will endanger human health even cause serious illness like leukemia or osteosarcoma [3].
Adsorption mechanism
FT-IR surface functional group analysis before and after adsorption of Sr2+
A Fourier infrared spectrometer was used to analyze the changes of the surface functional groups of SBAC-5, SBAC-6, and SBAC-7 before and after the adsorption of Sr2+.
It can be seen from Fig. S1 that SBAC-5, SBAC-6 and SBAC-7 have two round and blunt strong peaks at 3433 cm−1 and 3190 cm−1, respectively. They are caused by O–H stretching vibration of carboxyl group and phenolic hydroxyl group [25]. The peak intensity at 3190 cm−1 decreases significantly after Sr2+ adsorption, probably because the OH of the carboxyl group and phenolic hydroxyl group is consumed in the adsorption reaction. The peak at 1125 cm−1 may be C–O stretching vibration [25], which disappears obviously after adsorbing Sr2+, probably because the alcohol is reacted. Different from the adsorption of Pb2+ [26], the asymmetric tensile vibration of the carboxylate in the range of 1565–1665 cm−1 [36] produced insignificant changes in absorption peaks.
XPS analysis before and after adsorption of strontium
The XPS analysis of SBACs before and after the adsorption of Sr2+ as shown in Table S1 indicates that, after SBAC adsorbed Sr2+, O and Sr content increased, Ca content decreased. The increase of O indicates that it participated in the reaction. The mechanism analysis is shown in the supplementary material.
As for the decrease of Ca content, it is speculated that during the adsorption of Sr2+ by SBAC, other metal ions (like Ca2+, etc.) that had been adsorbed on the surface of SBAC were released into the solution through the exchange mechanism, Sr2+ is adsorbed on the surface of SBAC, so release of Ca2+, etc. was tested after SBAC adsorbed Sr2+ (“Release of other metal ions” section).
Release of other metal ions
Desorption of Ca2+, Mg2+ and Al3+ were tested in Sr2+ solution. The results are shown at Fig. S3. Linear slopes between the amount of Sr2+ adsorbed and the amount of desorbed Ca2+ are 0.717, 0.759, and 0.692 for SBAC-5, SBAC-6 and SBAC-7. For Mg2+, the slopes are between 0.05 and 0.1, all R2 were greater than 0.98. It can be concluded that approximately 75% to 85% of Sr2+adsorbed is indeed due to exchange with Ca2+ (70% to 75%) and Mg2+ (5% to 10%).
From the above analysis, the mechanism of SBAC removing Sr2+ from aqueous solution is mainly composed of the following two parts similar to another work [26]: (1) ion exchange, mainly the exchange of Sr2+ and Ca2+, (2) the complexation of oxygen-containing functional groups on the carbon surface with metal Sr2+, mainly hydroxyl.
Regeneration of SBAC after Sr2+ adsorption
In order to explore the reuse potential of SBAC after adsorbing Sr2+ and the recoverability of the adsorbed Sr2+, taking SBAC-7 as an example, a 0.1 M HCl solution was used to regenerate SBAC-Sr. The detailed experimental process is as follows: First, SBAC-Sr is regenerated with 0.1 M HCl solution. Afterwards, the regenerated SABC sample is rinsed with pure water until there is no significant change in pH. The sample is dried in the vacuum drying oven and continues to be used for adsorbing Sr2+. The experiment was repeated three times, and the test results are shown in Table 6.
As can be seen from Table 6, the adsorption capacity of SBAC for Sr2+ have gradually increased as the number of regenerations increases. It’s speculated that as result of the action of HCl, the metal ions previously adsorbed on the SBAC are released, providing more adsorption sites. The above results show that the adsorption capacity of SBAC after three-round repeated regeneration can complete recovery and even increase.
Possible secondary pollution analysis of SBAC
When SBAC is used to remove metal ions in actual water, it is necessary to consider whether SBAC itself as waste is hazardous when used in actual water. That is if other substances will release, causing secondary pollution to the water body. Therefore, the possible releases of organic matter and metal ions in SBAC were studied in this section.
Release of organic matter in SBAC
Considering that SBACs are the saturated AC used in the BAC process without pretreatment, the organic pollutants adsorbed on it may have a certain negative impact on its reuse. Therefore, release experiments of organic matter were respectively conducted in pure water, tap water, and actual river water (taken from the river water in the Peiyangn campus district of Tianjin University). The main ionic water quality indicators in different water bodies are shown in Table 7.
The release experiment measured changes in organic carbon (NPOC) by TOC-L CPN (Shimadzu, Co. LTD, detection limit is 0.010 mg L−1) through the same experimental method with Sr2+ batch adsorption experiments under the condition of SBAC content of 0.20 g L−1. The experiment lasted a total of 1320 min, and samples were taken at 60 min, 480 min and 1320 min respectively. The results are shown in Table 8.
From the data in Table 8, it can be seen that for different water bodies, there is a clear difference in the release of organic matter in SBAC, among which the release in laboratory pure water is more obvious, followed by the tap water, and the least in river water. That is, when SBAC is used for the removal of metals in actual water, the effect of organic matter release is negligible. However, the necessary pre-experiments must be carried out before application to rule out the possible comprehensive effects.
Judging from the release results of the three SBACs with different service years, the release value did not change much, and there was no obvious difference. As far as the release time is concerned, the results of 60 min, 480 min and 1320 min are also not significantly different, that is, the release of organic matter adsorbed by SBAC will not change significantly with the extension of use time.
Release of metal ions in SBAC
SBAC (0.20 g L−1, 2.0 g L−1) was added in 50 ml of pure water. After shaking for 12 h, the supernatant was taken to detect the concentration of metal ions, and the release was investigated. The results are shown in Table 9, which indicated the detected metal ions are mainly Ca2+, Mg2+, Al3+, and no release of harmful metals was detected. Considering that the concentrations of Ca2+, Mg2+, and Al3+ in tap water are 36.30, 13.05, and 0.128 mg L−1; the concentrations of Ca2+, Mg2+, and Al3+ in river water are 32.45, 44.85, and 0.16 mg L−1 (as shown in Table 9), the concentration is extremely small compared to its background concentration in actual water, which is within an acceptable range. In addition, when SBAC is used in actual water, because it is rich in cations, which will inhibit the release of metal ions, therefore, the amount of release is less than that in pure water. In summary, the release of metal in SBAC will not affect its application in actual water.
The foregoing analysis of release test can prove that when SBAC is used for the adsorption and removal of Sr2+ in actual water, the organic matter released by SBAC itself can be ignored; the metal released by itself is non-harmful metal and the content is small. In summary, the release of organic matter and metal ions adsorbed on the surface of SBAC shows that SBAC is feasible for the removal of heavy metals and radionuclides in actual water in terms of safety and secondary pollution.
Application of SBAC adsorption of Sr2+ in river water
The solution containing Sr2+ was prepared with river water (“Release of organic matter in SBAC” section) to simulate the actual water body contaminated with Sr2+. The sampled river water was first filtered through a 0.22 μm filter membrane to remove suspended solids and green algae before use. Using the same method as the previous adsorption isotherm experiment, the adsorption capacity of SBAC to Sr2+ in river water was investigated.
It can be seen from Fig. 7 that, compared with Ads in pure water, SBAC-5, SBAC-6 and SBAC-7 have a certain degree of reduction in Ads of Sr2+ in real river water. The maximum Ads of Sr2+ decreased from about 85% in pure water to 34–40% in river water. This can be explained by the aforementioned adsorption mechanism. The large amount of Ca2+, Mg2+ and other cations in the river affect the adsorption of SBAC. In addition, the large amount of organic matter in the river water (NPOC value of 50.42 mg L−1) may also affect ability to adsorb metal ions of SBAC [26]. Nevertheless, compared with other AC adsorbents from Table 4, SBAC still shows the best removal effect in actual river water. In addition, SBAC has nearly zero cost and does not require any pretreatment, achieving direct reuse of resources.
Considering that 90Sr and 137Cs usually occur simultaneously in radioactive wastewater [37,38,39], the adsorption effect of SBAC for Cs+, and the coexistence experiment of Sr2+ and Cs+ in river water are tested. It can be seen from Fig. 8a that the adsorption of Cs+ in river water is not regular and worse than Sr2+, which may be related to the different ionic radius and element properties of Sr2+ and Cs+. At the same time, the complex internal conditions of river water may affect the adsorption effect of Cs+, and the specific reasons need to be further explored. It is worth noting that the coexistence experiment of Sr2+ and Cs+ shows that when they coexist in river water, the removal rate of Sr2+ by SBAC is not affected by Cs+ (Fig. 8b). Therefore, when SBAC is applied to the treatment of wastewater containing Sr2+ and Cs+, other methods should be considered to remove Cs+.
Conclusions
In this work, three SBACs were investigated on removal of Sr2+ from water. There are somethings that draw people attention.
The adsorption of Sr2+ by SBAC has fast speed, high removal rate far above virgin AC, which shows the potential of SBAC for the removal of Sr2+. The removal mechanism of Sr2+ mainly has the following two points: Sr2+ exchanges with Ca2+ from SBAC and complexation of oxygen-containing functional groups on the surface (mainly hydroxyl groups and lactone group).
The leaching test shows the application of SBAC in water is safe. The regeneration experiment of SBACs shows their excellent regeneration performance. The desorption of SBACs shows that the amount of Sr2+ adsorbed by SBAC is much higher than the amount of desorption. The above verifications indicate the feasibility of SBAC being used in actual water. More importantly, SBAC has the advantages of low price, and good adsorption and regeneration performance. In short, the work has good practical application value.
References
Castrillejo M, Casacuberta N, Breier CF, Pike SM, Masqué P, Buesseler KO (2016) Reassessment of 90Sr, 137Cs, and 134Cs in the coast off Japan derived from the Fukushima Dai-ichi nuclear accident. Environ Sci Technol 50(1):173–180. https://doi.org/10.1021/acs.est.5b03903
Attallah MF, Rizk SE, Shady SA (2018) Separation of 152 + 154Eu, 90Sr from radioactive waste effluent using liquid–liquid extraction by polyglycerol phthalate. Nucl Sci Tech 29(6):84. https://doi.org/10.1007/s41365-018-0423-z
Ghandhi SA, Weber W, Melo D, Doyle-Eisele M, Chowdhury M, Guilmette R, Amundson SA (2015) Effect of 90Sr internal emitter on gene expression in mouse blood. BMC Genom. https://doi.org/10.1186/s12864-015-1774-z
Zhang Z, Gu P, Zhang M, Yan S, Dong L, Zhang G (2019) Synthesis of a robust layered metal sulfide for rapid and effective removal of Sr2 + from aqueous solutions. Chem Eng J 372:1205–1215. https://doi.org/10.1016/j.cej.2019.04.193
Wu L, Zhang G, Wang Q, La Hou GuP (2014) Removal of strontium from liquid waste using a hydraulic pellet co-precipitation microfiltration (HPC-MF) process. Desalination 349:31–38. https://doi.org/10.1016/j.desal.2014.06.020
Zhang L, Lu Y, Liu Y-L, Li M, Zhao H-Y, Hou L-A (2016) High flux MWCNTs-interlinked GO hybrid membranes survived in cross-flow filtration for the treatment of strontium-containing wastewater. J Hazard Mater 320:187–193. https://doi.org/10.1016/j.jhazmat.2016.08.020
Deli D, Law K, Liu Z, Crouch DJ, Livens FR, Yeates SG (2012) Selective removal of 90Sr and 60Co from aqueous solution using N-aza-crown ether functional poly(NIPAM) hydrogels. React Funct Polym 72(6):414–419. https://doi.org/10.1016/j.reactfunctpolym.2012.03.013
Wen T, Zhao Z, Shen C, Li J, Tan X, Zeb A, Wang X, Xu AW (2016) Multifunctional flexible free-standing titanate nanobelt membranes as efficient sorbents for the removal of radioactive (90)Sr(2 +) and (137)Cs(+) ions and oils. Sci Rep 6:20920. https://doi.org/10.1038/srep20920
Attallah MF, Borai EH, Hilal MA, Shehata FA, Abo-Aly MM (2011) Utilization of different crown ethers impregnated polymeric resin for treatment of low level liquid radioactive waste by column chromatography. J Hazard Mater 195:73–81. https://doi.org/10.1016/j.jhazmat.2011.08.007
Huang C-P, Lin T-Y, Chiao L-H, Chen H-B (2012) Characterization of radioactive contaminants and water treatment trials for the Taiwan Research Reactor’s spent fuel pool. J Hazard Mater 233–234:140–147. https://doi.org/10.1016/j.jhazmat.2012.07.009
Sahai N, Carroll SA, Roberts S, O’Day PA (2000) X-ray absorption spectroscopy of strontium(II) coordination: II. Sorption and precipitation at kaolinite, amorphous silica, and goethite surfaces. J Colloid Interface Sci 222(2):198–212. https://doi.org/10.1006/jcis.1999.6562
Karasyova ON, Ivanova LI, Lakshtanov LZ, Lövgren L (1999) Strontium sorption on hematite at elevated temperatures. J Colloid Interface Sci 220(2):419–428. https://doi.org/10.1006/jcis.1999.6474
Liang T-J, Hsu C-N, Liou D-C (1993) Modified Freundlich sorption of cesium and strontium on Wyoming bentonite. Appl Radiat Isot 44(9):1205–1208. https://doi.org/10.1016/0969-8043(93)90065-I
Jeong CH (2001) Mineralogical and hydrochemical effects on adsorption removal of cesium-137 and strontium-90 by kaolinite. J Environ Sci Health Part A Toxic Hazard Subst Environ Eng 6(36):1089–1099
Papachristodoulou CA, Assimakopoulos PA, Gangas NHJ (2002) Strontium adsorption properties of an aluminum-pillared montmorillonite carrying carboxylate functional groups. J Colloid Interface Sci 245(1):32–39. https://doi.org/10.1006/jcis.2001.7988
Cole T, Bidoglio G, Soupioni M, O’Gorman M, Gibson N (2000) Diffusion mechanisms of multiple strontium species in clay. Geochim Cosmochim Acta 64(3):385–396. https://doi.org/10.1016/S0016-7037(99)00324-5
Shawabkeh RA, Rockstraw DA, Bhada RK (2002) Copper and strontium adsorption by a novel carbon material manufactured from pecan shells. Carbon 40(5):781–786. https://doi.org/10.1016/S0008-6223(01)00198-1
Al-Jubouri SM, Curry NA, Holmes SM (2016) Hierarchical porous structured zeolite composite for removal of ionic contaminants from waste streams and effective encapsulation of hazardous waste. J Hazard Mater 320:241–251. https://doi.org/10.1016/j.jhazmat.2016.08.011
Zhang M, Gu P, Zhang Z, Liu J, Dong L, Zhang G (2018) Effective, rapid and selective adsorption of radioactive Sr2 + from aqueous solution by a novel metal sulfide adsorbent. Chem Eng J 351:668–677. https://doi.org/10.1016/j.cej.2018.06.069
Dong L, Hou L, Wang Z, Gu P, Chen G, Jiang R (2018) A new function of spent activated carbon in BAC process: removing heavy metals by ion exchange mechanism. J Hazard Mater 359(OCT.5):76–84
Chegrouche S, Mellah A, Barkat M (2009) Removal of strontium from aqueous solutions by adsorption onto activated carbon: kinetic and thermodynamic studies. Desalination 235(1):306–318. https://doi.org/10.1016/j.desal.2008.01.018
Moloukhia H, Hegazy WS, Abdel-Galil EA, Mahrous SS (2016) Removal of Eu3 + , Ce3 + , Sr2 + , and Cs + ions from radioactive waste solutions by modified activated carbon prepared from coconut shells. Chem Ecol 32(4):324–345. https://doi.org/10.1080/02757540.2016.1139089
Caccin M, Giacobbo F, Da Ros M, Besozzi L, Mariani M (2013) Adsorption of uranium, cesium and strontium onto coconut shell activated carbon. J Radioanal Nucl Chem 297(1):9–18. https://doi.org/10.1007/s10967-012-2305-x
Kubota T, Fukutani S, Ohta T, Mahara Y (2013) Removal of radioactive cesium, strontium, and iodine from natural waters using bentonite, zeolite, and activated carbon. J Radioanal Nucl Chem 296(2):981–984. https://doi.org/10.1007/s10967-012-2068-4
Andersson A, Laurent P, Kihn A, Prévost M, Servais P (2001) Impact of temperature on nitrification in biological activated carbon (BAC) filters used for drinking water treatment. Water Res 35(12):2923–2934
Dong L, Pan S, Liu J, Wang Z, La Hou, Chen G (2020) Performance and mechanism of Pb(II) removal from water by the spent biological activated carbon (SBAC) with different using-time. J Water Process Eng. https://doi.org/10.1016/j.jwpe.2020.101255
Sato I, Kudo H, Tsuda S (2011) Removal efficiency of water purifier and adsorbent for iodine, cesium, strontium, barium and zirconium in drinking water. J Toxicol Sci 36(6):829–834
International ASoTM (2014) Standard practice for determination of adsorptive capacity of activated carbon by aqueous phase isotherm technique, vol ASTM D3860-98. West Conshohocken, PA. https://doi.org/10.1520/d3860-98r14
Attallah MF, Borai EH, Allan KF (2009) Kinetic and thermodynamic studies for cesium removal from low-level liquid radioactive waste using impregnated polymeric material. Radiochemistry 51(6):622–627. https://doi.org/10.1134/s1066362209060113
Attallah MF, Abd-Elhamid AI, Ahmed IM, Aly HF (2018) Possible use of synthesized nano silica functionalized by Prussian blue as sorbent for removal of certain radionuclides from liquid radioactive waste. J Mol Liq 261:379–386. https://doi.org/10.1016/j.molliq.2018.04.050
Nayl AA, Ahmed IM, Abd-Elhamid AI, Aly HF, Attallah MF (2020) Selective sorption of 134Cs and 60Co radioisotopes using synthetic nanocopper ferrocyanide-SiO2 materials. Sep Purif Technol. https://doi.org/10.1016/j.seppur.2019.116060
Rizk HE, Attallah MF, Ali AMI (2017) Investigations on sorption performance of some radionuclides, heavy metals and lanthanides using mesoporous adsorbent material. J Radioanal Nucl Chem 314(3):2475–2487. https://doi.org/10.1007/s10967-017-5620-4
Attallah MF, Allan KF, Mahmoud MR (2015) Synthesis of poly(acrylic acid–maleic acid)SiO2/Al2O3 as novel composite material for cesium removal from acidic solutions. J Radioanal Nucl Chem 307(2):1231–1241. https://doi.org/10.1007/s10967-015-4349-1
Chegrouche S, Mellah A, Barkat M (2009) Removal of strontium from aqueous solutions by adsorption onto activated carbon: kinetic and thermodynamic studies. Desalination 235:306–318
Alarifi A, Hanafi H (2010) Adsorption of cesium, thallium, strontium and cobalt radionuclides using activated carbon. J At Mol Sci. https://doi.org/10.4208/jams.100809.112309a
Ganesh I, Sekhar PSC, Padmanabham G, Sundararajan G (2012) Influence of Li-doping on structural characteristics and photocatalytic activity of ZnO nano-powder formed in a novel solution pyro-hydrolysis route. Appl Surf Sci 259:524–537. https://doi.org/10.1016/j.apsusc.2012.07.077
Attallah MF, Hassan HS, Youssef MA (2019) Synthesis and sorption potential study of Al2O3ZrO2CeO2 composite material for removal of some radionuclides from radioactive waste effluent. Appl Radiat Isot 147:40–47. https://doi.org/10.1016/j.apradiso.2019.01.015
Hamed MM, Attallah MF, Metwally SS (2014) Simultaneous solid phase extraction of cobalt, strontium and cesium from liquid radioactive waste using microcrystalline naphthalene. Radiochim Acta. https://doi.org/10.1515/ract-2013-2200
Borai EH, Hilal MA, Attallah MF, Shehata FA (2008) Improvement of radioactive liquid waste treatment efficiency by sequential cationic and anionic ion exchangers. Radiochim Acta 96(7):441–447. https://doi.org/10.1524/ract.2008.1506
Acknowledgements
We thank Professor Jiang for the SBAC samples, and Tianjin University Testing Center of Environmental Quality for the testing of heavy metals.
Funding
This work was supported by Major Science and Technology Program for Water Pollution Control and Management in China [grant numbers 2015ZX07406006]; and the Independent Innovation Fund and Graduate Innovative Talent Training Project of Tianjin University, China [Grant Numbers 2018XZC-0080 and YC19056].
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dong, L., Wu, C., Han, Y. et al. Research on the application potential of spent biological activated carbon from BAC process to remove radionuclides Sr2+ from water. J Radioanal Nucl Chem 327, 1179–1190 (2021). https://doi.org/10.1007/s10967-021-07596-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07596-0