Abstract
The uranium in the radioactive wastewater produced by uranium conversion process is higher than the emission limit value, so it is of great significance to treat it to reduce its concentration. Nanofiltration technology and ion exchange technology were combined to treat uranium-containing wastewater and it can achieve the discharge standard. For the treatment system, uranium was firstly enriched in nanofiltration concentrate, and the concentrate entered the ion exchange system for selective adsorption of uranium. Because uranium exists in the form of complex cations or anions at different pH conditions, the use of cation–anion exchange resin tandem treatment technology can effectively and selectively adsorb almost all uranium. Both laboratory and engineering application results showed that the uranium concentration in the nanofiltration solution can be reduced to less than 50 μg/L after treating by the nanofiltration system for the wastewater containing 5–100 mg/L uranium. This work demonstrated that nanofiltration-ion exchange technology is practical and valuable for the treatment of acidic or alkaline uranium-containing waste liquid.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the rapid development of nuclear power in the world, the capacity of nuclear power units has shown a trend of doubling, and the uranium conversion industry has risen rapidly [1, 2]. However, the resulting problem is that a large amount of uranium wastewater has been produced. If this wastewater is discharged without control, uranium will enter the food chain through the biosphere and finally accumulate in the human body [3, 4]. The presence of uranium will cause radiation damage to the human body, which can cause acute or chronic poisoning and induce various diseases. The peaceful and green development of nuclear energy requires that uranium wastewater must be treated and discharged after reaching the standard, which has met the requirements of environmental monitoring and protection. The World Health Organization (WHO) recommended that the concentration of uranium (VI) in drinking water should be less than 2 μg/L. The United States Environmental Protection Agency (EPA) also stipulates a similar low concentration standard, usually the concentration of uranium in drinking water is limited to 30 μg/L or less. The emission standards for uranium-containing wastewater are set to 50 μg/L in China. These standards aim to control the potential impact of radioactive wastewater on the environment and public health. Therefore, it is necessary and urgent to develop new technologies for uranium-containing wastewater treatment.
The Savannah post-treatment plant in the United States adopts pH adjustment- microfiltration—mercury ion exchange—activated carbon column—reverse osmosis—ion exchange for the waste liquid/military legacy waste liquid from the post-treatment separation facility [5, 6]. The purified water is directly discharged, and the concentrated liquid is evaporated. The waste liquid treatment process was completed and put into use in 1998. The Nine Mile Point (NMP) nuclear power plant in the United States uses a two-stage filtration column (to remove suspended solids)—bag filtration—reverse osmosis prefiltration—reverse osmosis—photo oxidation—ion exchange—recycling and reuse (zero emissions) for environmental protection and public welfare treatment of ground flushing water and other waste [7].
Internationally, technologies such as microfiltration (MF), ultrafiltration (UF), and nanofiltration (NF) are gradually being applied in the field of radioactive wastewater treatment [8]. Compared with traditional treatment processes, membrane technology has many advantages, such as good effluent quality, energy savings, high decontamination coefficient, and stable and reliable operation [9,10,11]. In addition, ion exchange technology has been widely used in the treatment of uranium ions due to its excellent performance, high removal rate, small footprint, and low investment [12].
Therefore, this work innovatively combines nanofiltration technology and anion-cation exchange series technology to achieve qualified treatment and discharge of uranium containing waste liquid ([U] < 50 μg/L). The concentrated solution obtained after nanofiltration volume reduction and concentration is highly enriched uranium wastewater. Selective adsorption of uranium can be achieved by using anion cation exchange tandem technology to reduce the uranium content in the concentrated solution to 50 μg/L in China, the adsorbed tail liquid meets the discharge standard, and the adsorbed uranium can be recovered and reused through desorption. This work has created a new idea and method for the treatment of uranium-containing wastewater, which has great potential for industrial application.
Experimental and chemical
Experimental equipment
Nanofiltration device (Vontron Technology Co., Ltd, China), ion exchange resin column (DuPont, Co., Ltd, USA), polyamide (J&K Scientific Ltd) and tubular composite membrane (Porex Filtration Ltd). The accuracy of the first-stage nanofiltration membrane is 150 daltons, and the accuracy of the second-stage nanofiltration membrane is 300 daltons.
Materials and chemicals
Sodium carbonate, sodium chloride, hydrochloric acid and sodium hydroxide were purchased from Tianjin Damao Co., Ltd. China. SQD-74 and TP107 strong alkaline ion exchange resin, SQD-92 and SQD-813 weak alkaline ion exchange resins, TP207 and TP260 weak acidic ion exchange resin, SP112 strong acidic ion exchange resin were purchased from Tianjin Damao Co., Ltd. Details of all the resins used in the experiment are shown in Table 1. The 201 × 7 ion exchange resin used in the original wastewater treatment facility was used to adsorb the tail liquid. The uranium concentration was 5–100 mg/L and the pH was about 11. All of the resins were selected for ion exchange experiments.
Laboratory scale experiments
The uranium-containing wastewater in the experiment is first collected in the raw liquid tank, and then transported to the first level nanofiltration membrane module through a pressure pump after sampling and analysis. The concentrated solution of nanofiltration enters the concentrated solution tank, and the filtrate enters the primary clear solution tank. The filtrate in the first level clear liquid tank is then filtered through the second level nanofiltration membrane. The filtrate enters the second level clear liquid tank, and the concentrated solution enters the concentrated solution tank. The filtrate in the secondary clear liquid tank can also be returned to the primary clear liquid tank for repeated filtration. Finally, all experimental water can be returned to the original wastewater system for further uranium recovery. The process is shown in the Fig. 1.
The adsorption experiment of uranium by resin was carried out by dynamic column experiment. As shown in Fig. 2, the wastewater was adsorbed and separated by a column filled with resin, and the flow rate was controlled by a pump to ensure the same conditions. Four groups of solutions (pH 3, 4, 5, 6) were flowed through the TP260 cation exchange resin column, and the pH was adjusted to 7, 8, 9, 10 by Na2CO3 solution, and then they were adsorbed by the 07 anion exchange resin column. After 60 min of operation in each group, the effluent of the exchange column was sampled to determine the uranium content, and the system was washed and replaced after sampling. At the same time, in order to ensure the production cost, different eluents were used to regenerate the resin after saturated adsorption of uranium. The column after adsorption of uranium was rinsed with eluent and the effluent was collected for the detection of uranium concentration. The recovery rate of uranium was used to evaluate the regeneration performance of the resin.
Engineering application stage
The performance of the whole treatment system is evaluated by calculating the retention ratio (R = (1–C2/C1) × 100%), where C1 represents the uranium content in the versus wastewater of each level of nanofiltration membrane, C2 represents the uranium content in the permeate of various levels of NM. The uranium-containing wastewater with different uranium contents (5–100 mg/L) (pH = 8) was prepared, and the uranium concentration of the final supernatant was compared to verify the uranium interception effect of the nanofiltration system.
This experiment verifies the adsorption effect of uranium on all levels of exchange columns in the ion exchange system when the inlet pH of the cation exchange column is controlled at 3 and the inlet pH of the anion exchange column is controlled at 9.
Uranium measurement procedure: The concentrations of U in supernatants or solution were measured by Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES, PQ9000, Jena, Germany), prior to analysis, the instrument was washed and optimized to obtain an accurate measurement.
Results and discussion
Laboratory scale experiments
Results of nanofiltration experiment
The results showed that the initial uranium-containing wastewater (100 μg/L) was treated by 1# nanofiltration unit, and the uranium concentration was reduced to 0.93 μg/L (Table 2). After further treatment by 2# nanofiltration unit, the uranium concentration in the effluent was 0.042 μg/L, which was lower than the emission standard of 50 μg/L. Similarly, Torkabad et al. [13] used three commercial nanofiltration membranes (PES-2, NF-1 and NF-2) to selectively separate uranium from the bioleaching solution of low-grade uranium ore. The performance of the membrane under various operating conditions was evaluated by terminal and cross-flow filtration experiments. The experimental results showed that the three nanofiltration membranes could intercept uranium well. This result proves the effective retention ability of nanofiltration membrane to uranium, which is beneficial to the reduction of its concentration.
Ion exchange resin experiment
(1) The adsorption effect of ion exchange resin on uranium
According to Fig. 3, it can be found that none of the seven resins have reached 50 μg/L requirement. However, we can still find that the uranium concentration after resin TP260 and TP107 treatment is reduced to 0.45 and 0.149 mg/L, respectively. However, some resins including of SQD-74, SQD-92, SQD-813, TP207 and SP113 had certain adsorption capacities for uranium, compared with the other two resins, their weaker exchange capacity for uranium may be limited by the mismatch between the change of uranium species and its adsorption sites under this condition. Thus, Further experiments were conducted on the resin TP260 and TP107. The effluent (pH ~ 8–9) after adsorption of TP260 resin in the above experiment was collected, and the pH was adjusted to 10 with Na2CO3, and then further adsorbed by TP107 resin. The results of adsorption tail liquid analysis showed that the uranium concentration in the solution was 25.6 μg/L, which achieved the expected uranium enrichment effect and was lower than the emission standard. According to the Botha et al. report, an anionic resin was used for groundwater containing uranium treatment, the results showed about 99.5% removal of uranium from an effluent with a pH of 6.5 in a responsible system containing a large number of coexisting ions [14]. In comparison, the ion exchange resin we selected has a better ability to remove uranium.
The results show that it is difficult to achieve the requirement that the uranium content in the effluent is less than 50 μg/L by using anion exchange resin or cation exchange resin alone to adsorb uranium ions in uranium-containing wastewater. The first reason is that the concentration of uranium ions in the uranium-containing wastewater to be treated is too low, resulting in insufficient adsorption capacity of the resin to uranium ions [15]. The second reason is that when pH is 3 and 10, the valence state of uranium in uranium-containing wastewater is not unique, and there are still trace amounts of uranium that cannot be adsorbed by the corresponding cation and anion exchange resins. Through this experiment, the feasibility of the process route of treating uranium-containing wastewater in series with cation and anion exchange resins was verified. This method is easier to achieve the environmental protection requirements of effluent less than 50 μg/L than using a single ion exchange resin [16].
(2) Effect of pH on uranium adsorption efficiency in ion exchange systems
Figure 4 shows the uranium content data of the adsorption tail liquid at different pH values. The results showed that the uranium containing wastewater with a pH of 3 was adsorbed by a cation exchange resin, the wastewater pH was adjusted to 9 and 10 with Na2CO3, and then adsorbed again with an anion exchange resin, the uranium content in the wastewater can be reduced to 42 and 23 μg/L (< 50 μg/L) for the effluent with pH of 9 and 10, respectively. This indicates that uranium exists in different forms in solutions of different pH, and deep recovery of uranium can be achieved through a series of cation anion exchange columns.
In order to obtain the maximum adsorption capacity and desorption parameters of TP260 resin in the treatment of real uranium-containing wasteswater, the column experiment was carried out to investigate the dynamic adsorption behavior. The results showed that the resin column could treat about 750 mL solution when the uranium concentration was 2 g/L, so as to obtain the maximum uranium adsorption capacity of the TP260 resin was 26 mg/g. At the same time, 10% HCl and 10% Na2CO3 solution were used as eluents, respectively, the results showed that more than 95% of uranium could be eluted from the column using 10% Na2CO3 solution as eluent.
Engineering application-experimental results and discussion
(1) Development of nanofiltration system
The nanofiltration device is equipped with four levels of NM (Fig. 5), among which the selection accuracy of 2#, 3#, and 4# nanofiltration is 300 Dalton NM, and the selection accuracy of 1# nanofiltration is 150 Dalton NM. The power between the NM is provided by a variable frequency high-pressure pump. The clear liquid phases of primary nanofiltration (1#NM), secondary nanofiltration (3#NM), and tertiary nanofiltration (4#NM) are connected in series to separate the uranyl ions and their complex anions in uranium containing wastewater through tertiary filtration, layer by layer interception, ensuring that [U] < 50 μg/L in the outlet clear liquid. Concentrated nanofiltration (2# NM) further concentrates the concentrated solution after the first stage nanofiltration, returns the clear solution to the 1# nanofiltration versus wastewater tank, and further removes uranium from the concentrated solution through the ion exchange system. 2# and 3# nanofiltration concentrated liquids are partially diverted to their respective versus wastewater tanks, and some are returned to the 1# nanofiltration versus wastewater tank to ensure relatively stable uranium concentration in each level of water tank. The efficiency of the system for concentrated water entering the ion exchange system is about 10–30%.
(2) Development of ion exchange system
The ion exchanger adopts a floating bed form, during operation, the inlet water enters the floating bed from the bottom, passes through the lower water distribution plate, and evenly enters the bed layer. The entire resin layer is lifted in a uniform and dense state by the rising water flow, at the same time, the water flow completes the ion exchange reaction with the resin layer during the upward flow and flows out of the ion exchanger from the top. After the resin layer becomes saturated, the cleaning water and regenerant enter from the upper part, pass through the upper water distribution plate, and evenly flow downwards through the resin layer for cleaning and regeneration. In order to distribute water more evenly and prevent accidental loss of resin, water distribution plates are installed at the lower and upper parts of the chamber, and ion exchange resin special filter caps are installed on the perforated plates. This ensures that the resin with normal particle size will not lose, and water flow can pass through normally. It also clamps other small impurities inside the resin tower to ensure cleanliness inside the tower. At the same time, the water flow is effectively and evenly distributed during the backwashing process of the resin tower. This maintains the stable distribution and flow state of the resin in the tower and improves the backwashing efficiency.
The ion exchange column for adsorption is divided into two stages. The first stage ion exchange column is a cation exchange column packed with macroporous crosslinked polyacrylic acid series exchange resin with chelating aminomethyl phosphate group and weak acid cation, and the second stage ion exchange column is an anion exchange column packed with macroporous cross-linked polyacrylic acid series quaternary ammonium strong base anion exchange resin. Each stage consists of two units. During operation, each stage can operate as a single column or in series, and the operation mode is determined based on the uranium content of the versus wastewater [17, 18]. Compared with the traditional gel-type resin, the macroporous resin is easier to adsorb large molecular weight substances, and the adsorption efficiency of uranium is better [19]. The secondary adsorption effect after regeneration is good, and it is not easy to be blocked by impurities. The resin channel has strong anti-pollution ability.
The ion exchange device is used to treat nanofiltration-concentrated solution (Fig. 6). Before the wastewater enters the first level ion exchange column, the H2SO4 solution is added by the dosing pump through the back-pressure valve. The pH of the wastewater is adjusted to 2–5 in the pipeline mixer before entering the column, and then adsorbed by the cation exchange resin. The effluent from the first level ion exchange column is collected by the pH adjustment reaction tank, where alkali is introduced to modify its pH level. After adjusting the wastewater pH to 9–11, it overflows to the adjustment tank and is then transported to the second level ion exchange column by a transport pump, where it is adsorbed by an anion exchange resin. The effluent from the secondary ion exchange column is collected by the neutralization tank and overflowed to the discharge tank before being discharged.
Analysis of nanofiltration experiment results
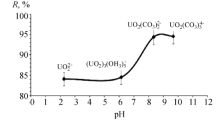
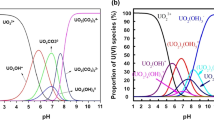
The experiment was conducted by configuring acidic and alkaline wastewater with a uranium content of 6–8 mg/L to calculate the retention ratio of various levels of NM, and the results were shown in Table 3. After the ion exchange system, the uranium-containing wastewater is concentrated to a smaller volume, and the uranium concentration is significantly increased. It revealed that the single stage nanofiltration membrane has a high uranium retention ratio of 85.54–91.59% in the treatment of alkaline wastewater containing uranium at concentrations ranging from 0.083 to 14.63 mg/L. While for acidic uranium containing wastewater with a concentration of 0.182–13.82 mg/L, the single stage nanofiltration membrane has a uranium retention ratio of 84.29–89.90%, which is slightly lower than that in alkaline environments. However, the uranium content in the third stage, clear solution is less than 50 μg/L, the retention ratio can still meet the process requirements. There are two reasons why the retention rates of alkaline and acidic uranium containing wastewater are different. One is that uranium has a large molecular weight, and the nanofiltration membrane has a pore size of about 1 nm, which has the best retention efficiency for substances with molecular weights ranging from 200 to 1000 Daltons. The second reason is that the nanofiltration membrane carries negative charges in water, while uranium in alkaline wastewater mainly exists in the form of anionic complexes UO2(CO3)22− and UO2(CO3)34−. The same charges repel each other, and the nanofiltration membrane prevents UO2(CO3)22− and UO2(CO3)34− from approaching it, thereby limiting its penetration through the membrane surface, resulting in a higher removal ratio [20]. However, uranium in acidic wastewater mainly exists in the form of UO22+, and the charge of the nanofiltration membrane is not conducive to its retention of uranium, resulting in a decrease in removal efficiency.
Analysis of ion exchange system experimental results
The inlet pH of the cation exchange column is controlled at 3, and the inlet pH of the anion exchange column is controlled at 9. The analysis data of the effluent from each level of the exchange column in the ion exchange system are shown in the Table 4. The results show that over 99% of uranium in wastewater is adsorbed by cation exchange resin, but the effluent cannot meet the standard. The residual nuclides are adsorbed by anion exchange resin and reach the emission standard of is consistent with the conclusion of the small experiment.
Conclusion
-
(1)
The nanofiltration membrane has a high retention ratio for uranium. After treating 8 mg/L of uranium-containing wastewater by the nanofiltration system, the uranium concentration in the supernatant is less than 50 μg/L.
-
(2)
The existence forms of uranium under different pH conditions can be divided into cationic and anionic forms. The series treatment technology of cation/anion exchange resins can effectively selectively adsorb different forms of uranium, making the uranium concentration in the tail liquid adsorbed by the exchange resin less than 50 μg/L.
-
(3)
The desorption agent for TP260 ion exchange resin is 10% sodium carbonate solution, and the optimal desorption agent for TP107 ion exchange resin is 10% sodium chloride and hydrochloric acid solution.
-
(4)
Through laboratory experiments and pilot plant validation, the combined treatment technology of nanofiltration ion exchange can achieve concentration, volume reduction, and deep adsorption of uranium containing wastewater from uranium purification and conversion production lines, so that the uranium concentration in the discharged water can meet the national discharge standards.
References
Abney CW, Mayes RT, Saito T, Dai S (2017) Materials for the recovery of uranium from seawater. Chem Rev 117(23):13935–14013
Cumberland SA, Douglas G, Grice K, Moreau JW (2016) Uranium mobility in organic matter-rich sediments: a review of geological and geochemical processes. Earth-Sci Rev 159:160–185
Beni AA (2021) Design of a solar reactor for the removal of uranium from simulated nuclear wastewater with oil-apatite ELM system. Arab J Chem 14(2):102959
Chen L, Liu J, Zhang W, Zhou J, Luo D, Li Z (2021) Uranium (U) source, speciation, uptake, toxicity and bioremediation strategies in soil-plant system: a review. J Hazard Mater 413:125319
Seo HW, Oh JY, Shin WG (2021) Proposal for the list of potential radionuclides of interest during NPP site characterization or final status surveys. Nucl Eng Technol 53(1):234–243
Danko B, Dybczyński RS, Samczyński Z, Gajda D, Herdzik-Koniecko I, Zakrzewska-Kołtuniewicz G, Chajduk E, Kulisa K (2017) Ion exchange investigation for recovery of uranium from acidic pregnant leach solutions. Nukleonika 62(3):213–221
Rodríguez-Penalonga L, Yolanda Moratilla Soria B (2017) A review of the nuclear fuel cycle strategies and the spent nuclear fuel management technologies. Energies 10(8):1235
Costa Peluzo BMT, Kraka E (2022) Uranium: the nuclear fuel cycle and beyond. Int J Mol Sci 23(9):4655
Jessica VC (2016) Separation of actinides from spent nuclear fuel: a review. J Hazard Mater 318(15):266–281
Yu R, Zhang X, Lu Y, Chen W, Chen X, Li L (2022) Advanced amidoximated polyethylene nanofibrous membranes for practical uranium extraction from seawater. ACS Sustain Chem Eng 10(37):12307–12318
Yu R, Lu Y, Zhang X, Chen W, Chen X, Li L (2022) Amidoxime-modified ultrathin polyethylene fibrous membrane for uranium extraction from seawater. Desalination 539(1):115965
Thakur P, Mulholland GP (2011) Determination of Pu, Am, U and Cs in large soil samples in the vicinity of the USDOE Waste Isolation Pilot Plant. J Radioanal Nucl Chem 288:499–506
Torkabad MG, Keshtkar AR, Safdari SJ (2018) Selective concentration of uranium from bioleach liquor of low-grade uranium ore by nanofiltration process. Hydrometallurgy 178:106–115
Botha M, Bester L, Hardwick E (2009) Removal of uranium from mine water using ion exchange at Driefontein mine, Water Institute of Southern Africa & International Mine Water Association: Proceedings, International Mine Water Conference. 382–391
Bai J, Ma X, Gong C, Chen Y, Yan H, Wang K, Wang J (2020) A novel amidoxime functionalized porous resins for rapidly selective uranium uptake from solution. J Mol Liq 320(15):114443
Wu X, Huang Q, Mao Y, Wang X, Wang Y, Hu Q, Wang H, Wang X (2019) Sensors for determination of uranium: a review. TrAC Trend Anal Chem 118:89–111
Liu Z, Liu D, Cai Z, Wang Y, Zhou L (2018) Synthesis of new type dipropyl imide chelating resin and its potential for uranium(VI) adsorption. J Radioanal Nucl Chem 318:1219–1227
Massoud A, Masoud AM, Youssef WM (2019) Sorption characteristics of uranium from sulfate leach liquor by commercial strong base anion exchange resins. J Radioanal Nucl Chem 322:1065–1077
Campbell EL, Levitskaia TG, Fujimoto MS, Holfeltz VE, Chatterjee S, Hall GB (2018) Analysis of uranium ion exchange resin from the 200 west pump-and-treat facility. Pacific northwest national lab.(PNNL), richland, WA (United State)
Ladeira AC, Goncalves JS, Morais CA (2011) Treatment of effluents from uranium oxide production. Environ Technol 32(2):127–131
Acknowledgements
The financial support from Gansu Industrial Support Plan of colleges and Universities (2022CYZC-06) and Science and Technology Projects of Gansu Province (No. 23ZDFA014) are gratefully appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Geng, L., Chang, B., Li, T. et al. Treatment of wastewater by nanofiltration-ion exchange technology in uranium conversion process. J Radioanal Nucl Chem (2024). https://doi.org/10.1007/s10967-024-09762-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10967-024-09762-6