Abstract
A zirconium molybdophosphate composite was designed for the selective recovery of uranium ions. The synthesized composite was well-characterized using SEM, EDX, FT-IR and XRD. U(VI) ions recovery from their aqueous solution by the studied composite was investigated in terms of adsorption isotherm, and thermodynamics. The composite is highly efficient and selective in U(VI) ions capturing with Langmuir adsorption capacity of 192.3 mg/g. Results showed that the composite is highly efficient in removal for U(VI) ions from Abu Rusheid leach liquor. As well as, it exhibits long-term stability, reusability, and fast kinetic in capturing of uranyl ions from leach liquor.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Uranium is a radioactive element with an average abundance of 2–4 μg/g in the Earth’s crust. Uranium is a vital element for the nuclear energy industry [1, 2]. Uranium and their compounds are potential occupational carcinogens, because of their toxicity and radioactivity nature. Uranium can pollute the environment through mining and different nuclear activities. Therefore, there is a growing interest in uranium extraction from its sources and from the environment in view of the economy and environmental tasks [1,2,3].
There are several technologies used in uranium extraction include chemical precipitation, solvent extraction, solid/liquid extraction etc. [2, 3]. Solid/liquid of great importance due to its simplicity, economy and environmental advantage [4,5,6]. Solid/liquid extraction includes the transfer of uranium ions from its bearing solutions to the solid sorbent through their interaction/adsorption on the adsorbent functional groups. The transfer is stimulated by the selection of appropriate optimal conditions in the system (liquid-phase, analyte and solid-phase) [2, 7]. The analyte after adsorption on the solid phase is either eluted with a suitable elute or further analyzed. There are several solid adsorbents were used in uranium extraction like graphene oxide [2, 4], petroleum pitch-based porous aromatic frameworks [5], polypropylene [6], silica [7], polystyrene [8], glycidylmethacrylate [9], chitosan [10], activated carbon [11], etc.
Newly growing demand to find nature adsorbents to replace petrochemical products by natural adsorbents, in this respect, inorganic composites have greater importance because of their advantages such as physicochemical stability, high solubility and a relatively high sorption capacity due to their tendency to form complexes with a large number of metals. Many of the inorganic composites have been used to recover uranium from its bearing solutions such as zirconium phosphate [12], zirconium and titanium phosphates [13], zirconium-molybdopyrophosphate-tributyl phosphate [14], and silica–zirconium phosphate-phosphoric acid [15]. Strategies oriented towards the development of environmentally friendly and cost-effective purification adsorbents aimed at better extraction of uranium ions. In this concern, zirconium molybdophosphate composites are important inorganic materials as it exhibited long-term stability, high selectivity, reusability, and fast kinetic adsorption of various metal ions in our environment, geological and industrial samples.
In this paper, zirconium molybdophosphate composite was synthesized, characterized and tested for the selective recovery of uranyl ions from Abu Rusheid leach liquor (Abu Rusheid area, south Eastern Desert, Egypt). The adsorbent was characterized using FT-IR, SEM, EDX, and XRD techniques. The adsorption condition was optimized by varying experimental parameters to investigated the adsorption process in terms of, equilibrium, isotherm, kinetic, thermodynamics, and practical view.
Experimental
Reagents and instruments
U(VI) standard solution is Sigma-Aldrich product. Uranium ions were analyzed by the oxidimetric titration method and by confirmed spectrophotometericlly by Arsenazo III method [16]. Other elements were analyzed by ICP-OES, XRF, FT-IR, XRD, and SEM, (Supplementary Information, SI).
Synthesis of (zirconium molybdophosphate) composite
Zirconium molybdophosphate composite can be prepared by mixing one gram of sodium molybdate (Na2MoO4) and dipotassium hydrogen phosphate (K2HPO4). The mixture was dissolved in 100 mL of water then, zircon chloride (0.2 M) was added. The precipitate formed was re-dissolved in a 1.0 M HCl, followed by the addition of 30 mL tributyl phosphate, after 4 h. the product was collected, washed and dried for 8 h (SI).
Characterization of (zirconium molybdophosphate) composite
The analyses of trace elements were analyzed using ICP-OES., 1976). The zirconium molybdophosphate composite was characterized by X-ray fluorescence (XRF, Philp’s experimental unit with automatic sample changer PW 1510, JEOL-JSX-3222), Fourier transform infrared spectrometer (FT-IR, Thermo Scientific-NICOLET iS10, USA), X-ray diffraction (XRD, Phillips X-ray, PW3710, fitted with a generator PW 1830 and Cu target tube PW 223/20 at 40 kV and 30 mA), and by scanning electron microscope (SEM, XL30 Philips type).
Preparation of Abu Rusheid sample
The studied sample was subjected to uranium leaching (− 200 mesh size ground sample, 3 M H2SO4 acid as a leaching agent, 1/5 solid/liquid ratio, 200 rpm, and 0.5 h). The obtained leach liquor filtered, then, its uranium content was measured.
Adsorption procedures
The retention of U(VI) on the prepared zirconium phosphate composite as a function of pH (1–7), time (5 to 150 min), initial uranium concentration (50 to 400 mg/L), temperature (25–100 °C), and sorbent dose (2 to 20 mg) were investigated using 10 mL of uranium solution at optimum conditions of other factors. Three mineral acids (0.10 to 1.0 M) were examined to eluate uranium from the uranium loaded composite (SI).
The uranium uptake capacity (qe, mg/g), adsorption efficiency (E%) and distribution coefficient (Kd) were calculated from the following equations [17].
where m, V, Co, and Ce are composite weight (g), the volume of the solution (L), the initial and the final uranium concentrations (mg/L), respectively.
Results and discussions
Characterization of (zirconium molybdophosphate) composite
X-ray fluorescence (XRF)
The prepared composite was identified by X-ray fluorescence (Fig. S1), the analysis illustrates that the prepared composite contains ZrO2, P2O5, MoO3 and K2O with a percentage of 70.8709, 17.1126, 10.10, and 1.9165, respectively. From the latter results, we prove that the main element oxides are ZrO2, P2O5 and MoO3 constituents of the zirconium molybdophosphate composite.
Fourier transforms infrared spectrometer
FTIR spectrum is a useful tool to identify molecular and functional groups [18]. The spectrum of the prepared zirconium molybdo-phosphate composite (Fig. S2) shows peaks at 600, 790, 886 and 930 cm−1 correspondings to PO43−. The peak at 981 cm−1 represented P=O stretch. One band at 1259 cm−1 is attributed to (RO)3 P=O. Two beaks at 1465 and 1600 cm−1 are related to PO2. The band at 2878 cm−1 is attributed to O=P–OH, while the peak at 2962 cm−1 is corresponding to P-OH. Also, at 3588 cm−1 represented broadband involving OH stretch form. After uranium adsorption, the bands of PO43− were shifted to 615, 793 and 863 cm−1 and the two peaks at 1259 and 930 cm−1 were disappeared. Also, six bands at 981, 1465, 1600, 2878 2962 and 3588 cm−1 were shifted to 975, 1458, 1648, 2876, 2961 and 3596 cm−1. The major contributions from the phosphate groups have been changed due to the reaction with uranium ions.
Scanning electron microscope (SEM)
SEM images indicate that the surface of zirconium molybdophosphate composite has a deep hole of different sizes, (Fig. S3). While, the image of the surface after adsorption of uranium shows that, the surface is amorphous to be growing in clumps and coating cracks. The chemical composition of zirconium molybdophosphate given by EDX analyses is P 34.99%, K 7.69%, Ca 2.83%, and Zr 54.49%, where this composition after uranium adsorption is P 25.65%, U 23.78%, K 4.09%, Ca 0.96%, and Zr 45.53%.
X-ray diffractions (XRD)
Comparative XRD patterns of the free zirconium molybdophosphate are illustrated in Fig. S4a and the uranium loaded one is shown in Fig. S4b. The diffraction peak of zirconium molybdophosphate with uranium indicated at 5.180°, 52.215°, 70.545°, and 83.280° was shifted to higher values, confirming uranium adsorption on the composite.
Effect of pH
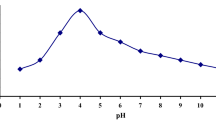
Figure 1 shows the dependence of U(VI) adsorption on pH on by zirconium phosphate composite, the results indicate that U(VI) adsorption efficiency increases with increasing the pH to 3.5 (adsorption efficiency = 90%, and qe = 180 mg/g) and then adsorption efficiency decreases with the further pH increasing. The results show that the prepared adsorbent has a good absorption capacity compared to other adsorbents (Table S1). When the solution is acidic, the adsorption on the protonated adsorbent surface is facilitated by electrostatic attraction of the uranium anion species in solution with the positively charged surface. At pH > 3.5, the protonation is decreased and the surface begins to polarized and hence carry a negative charge which electrostatic repulsed with uranium anions, so the adsorption capacity is slightly reduced. The suggested mechanism is electrostatic interaction between the adsorbent and uranium ion species.
Effect of time
The effect of time (Fig. 2) clearly shows that uranium adsorption efficiency increases with increasing the contact time until it reaches a plateau after 30 min. Moreover, the U(VI) adsorption efficiency was increased from 79% (5 min) to 90% (180 mg/g). The rapid initial uptake of uranium by zirconium molybdophosphate may be due to the availability of completely vacant adsorption sites at the beginning of the sorption process. After a rapid initial uptake, there was a transitional phase in which the rate of uptake was slow with uptake reaching almost a constant value at 30 min. The low equilibrium time indicates a great efficiency of the zirconium molybdophosphate adsorbent towards U(VI)ions from their aqueous solution.
Effect of initial uranium concentration
The effect of U(VI) concentration, expressed in terms of both adsorption efficiency and maximum uptake (Fig. 3), reveals that the maximum uptake (mg/g) of the adsorbent improved with increasing U(VI) concentration, while, adsorption efficiency (%) is decreased.
The decreasing in adsorption efficiency (%) may be due to the fact that the lower U(VI) concentrations in the sorption medium have greater availability for sorption sites, and this availability decrease as uranium ions increase (increase in the ratio of adsorbate per mass unit of the adsorbent), so adsorption efficiency (%) decrease while maximum uptake (mg/g) increase.
Effect of temperature
On the other hand, the adsorption capacity decreases with the increase in temperature (Fig. 4). It is clear that the adsorption efficiency of uranium sharply decreased (from 90 to 40%) with an increase in the temperature from 25 to 100°C, respectively. The negative effect of temperature on the adsorption process indicating that the process may be exothermic in nature.
Effect of composite dose
The composite dose can affect the sorbent-sorbate equilibrium of the system (Fig. 5). Uranium adsorption efficiency increased from 70 to 90% with an increased dose from 2 mg to 10 mg and then remains constant. The higher composite dose provides more available sorption sites and hence provides higher adsorption efficiency (%). Therefore, the optimum composite dose was chosen as 10 mg of composite per 10 mL of uranium solution (Fig. 5).
Desorption and regeneration of the prepared (zirconium molybdophosphate)
Desorption studies will help to regenerate the spent composite. Uranium desorption from loaded zirconium molybdophosphate was studied by H2SO4, HCL, and HNO3 (SI). The elution efficiency was found to be increased by increasing the acid concentration, a maximum elution efficiency of 99% was obtained by using 0.25 M H2SO4 or 0.75 M HNO3.
The adsorption–desorption cycle was repeated five times using 0.25 M H2SO4. After five cycles the adsorption efficiency of (zirconium molybdophosphate) composite was decreased from 90 to 85% and the desorption decreased from 99 to 90%. Therefore, 0.25 M H2SO4 acid could regenerate the composite effectively.
Adsorption equilibrium studies
Generally, adsorption isotherms are used to understanding the adsorption mechanism [19]. The most frequent models used are the Langmuir and Freundlich models. The Langmuir model represents a monolayer coverage adsorption process (Langmuir [20]) expressed by the following equation [20, 21]:
where b and qmax are Langmuir constant and the maximum capacity (mg/g). The Langmuir plots of (Fig. 6) show higher correlation coefficient values and Table 1 shows a maximum capacity (qmax) much close to experimental capacity this indicates that the U(VI) ion adsorption process is fitted with Langmuir isotherm model confirming the monolayer coverage of uranium onto the adsorbent and indicate the favorability of the chemical adsorption process.
Freundlich isotherm model suggested a multilayer heterogeneous adsorption process [17, 21, 22]:
Kf and n are constants related to the adsorption capacity and energy of adsorption, respectively. Freundlich plots (Fig. S5), and adsorption parameters (Table 1) show that the values of Kf (mg/g) are lower than the experimental capacity of U(VI) on the composite indicated that the adsorption process is not a heterogeneous the experimental data does not fit Freundlich isotherm. Also, The Freundlich plot gave a slope less than unity indicating the nonlinear sorption behavior with U(VI) in the concentration range studied.
Adsorption kinetic studies
Two kinetic models were used to analyze the adsorption of U(VI) onto the composite. Firstly, the pseudo first order model was applied according to Eq. 6 [21, 23]:
where qt and qe (mg/g) are the uranium adsorbed at time t (min) and at equilibrium, respectively. k1 is the adsorption rate constant (1/min). The adsorption rate constant (k1) can be determined experimentally by the plotting of Log (qe − qt) versus t (Fig. S6). From the data in Table 2 (the sorption capacity of 8.45 mg/g, besides R2 value of 0.8182) it can conclude that the adsorption processes don’t fit a pseudo first order kinetic model.
The second model is pseudo second order model which is given by Eq. 7 [21, 24, 25]:
The straight lines of plots of t/qt against t give the slope 1/qe and the intercept 1/k2q2e (Fig. 7). The calculated value of qe is found to be 181.8 mg/g and the correlation coefficient (R2) equals 0.9997, respectively (Table 2). The obtained qe is perfectly close to the experimental one, indicated that the process is mainly controlled by the chemical adsorption mechanism on the composite active sites [26]. The rate of adsorption is less controlled by intra-particle diffusion due to the bulky size of U(VI) ions that implies that adsorption of U(VI) is dependent on the concentration of both the metal ions and active sites concentrations.
Thermodynamic of the uranium adsorption process
Thermodynamic parameters associated with the adsorption (enthalpy change (∆H, KJ/mol), entropy change (∆S, KJ/mol K) and Gibbs free energy (∆G, KJ/mol)) were calculated by Eqs. 8–10 [21, 26]:
where Kd is the adsorption equilibrium constant (L/g) and R is the universal gas constant (8.314 J/mol K). By plotting a graph of Log Kd versus 1000/T, K−1, the values ∆H and ∆S can be calculated from the slope and intercept, respectively (Fig. 8).
All thermodynamic parameters obtained from the plotted graph are listed in (Table 3). The negative values of ∆H suggest the exothermic nature of the adsorption of uranium onto the prepared composite. The value of ∆G suggests a spontaneous adsorption reaction.
Uranium adsorption from the Abu Rusheid leach liquor using the prepared zirconium molybdophosphate composite
Table 4 shows the analyses of major and trace elements in the studied sample. The Abu Rusheid sample has uranium concentration assaying 2000 mg/kg.
In this case, 1 kg of the Abu Rusheid sample with particle size -200 mesh was subjected to acid leaching at the following optimum conditions: 3.0 M H2SO4 acid with ratio 1:5 (S/L) for 0.5 h. at room temperature, with leaching efficiency (90%). After that, the solution was filtered then the uranium was measured in leach liquor. The obtained leach liquor has 360 mg/L for uranium.
The applied experiment was achieved under the previously optimum conditions (pH 3.5, 200 rpm stirring speed, 30 min contact time at room temperature) by mixing 5L of the studied leach liquor with 1.8 g of the prepared zirconium molybdophosphate composite. U(VI) was eluted from the loaded prepared composite and then it was precipitated using sodium hydroxide at pH 7 as sodium diuranate (Na2U2O7). The final product was dried at 110 °C for 1 h, the uranium content was found to assay about 67.5% and the purity of it was 90%. The chemical composition of uranium precipitate as given by EDX analyses is P 10.53%, S 0.19%, K 11.09, Ca 6.48%, Fe 1.94 and U 69.76%. Finally, a schematic flow sheet for the precipitation of uranium from the Abu Rusheid area was presented in Fig. 9.
Conclusions
Zirconium molybdophosphate composite was successfully synthesized for the selective extraction uranium from its sources under optimized conditions (pH 3.5, 200 rpm, 30 min. The maximum uptake capacity of the prepared composite has attained 180 mg/g where the Langmuir adsorption capacity of 192.3 mg/g. Good reusability of zirconium molybdophosphate also indicated that the excellent adsorption stability for uranium recovery. Finally, the optimized factors have been carried out for uranium adsorption from Abu Rusheid leach liquor, Abu Rusheid area, south Eastern Desert, Egypt. The final precipitate of uranium was confirmed by chemically and by the EDX techniques.
References
Wang J, Liu J, Hongchun L, Chen Y, Xiao T, Song G, Chen D, Wangn C (2017) Uranium and thorium leachability in contaminated stream sediments from a uranium mine site. J Geochem Explor 176:85–90
Atia BM, Gado MA, Abd El-Magied MO, Elshehy EA (2019) Highly efficient extraction of uranyl ions from aqueous solutions using multi-chelators functionalized graphene oxide. Sep Sci Technol. https://doi.org/10.1080/01496395.2019.1650769
Xu M, Wang T, Gao P, Zhao L, Zhou L, Hua D (2019) Highly fluorescent conjugated microporous polymers for concurrent adsorption and detection of uranium. J Mater Chem A 7:11214–11222
Li S, Yang P, Liu X, Zhang J, Xie W, Wang C, Liu C, Guo Z (2019) Graphene oxide based dopamine mussel-like cross-linked polyethylene imine nanocomposite coating with enhanced hexavalent uranium adsorption. J Mater Chem A 7:16902–16911
Wang T, Xu M, Han X, Yang S, Hua D (2019) Petroleum pitch-based porous aromatic frameworks with phosphonate ligand for efficient separation of uranium from radioactive effluents. J Hazard Mater 368:214–220
Yang S, Ji G, Cai S, Xu M, Hua D (2019) Polypropylene nonwoven fabric modified with oxime and guanidine for antibiofouling and highly selective uranium recovery from seawater. J Radioanal Nucl Chem 321:323–332
Abd El-Magied MO, Dhmees AS, Abd El-Hamid AAM, Eldesouky EM (2018) Uranium extraction by sulfonated mesoporous silica derived from blast furnace slag. J Nucl Mater 509:295–304
Skripchenko SY, Titova SM, Smirnov AL, Rychkov VN (2019) Uranium sorption from productive solutions prepared by carbonate leaching from peat ore. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-019-06751-y
Sadeek SA, El-Sayed MA, Amine MM, Abd El-Magied MO (2014) Selective solid-phase extraction of U(VI) by amine functionalized glycidyl methacrylate. J Environ Chem Eng 2:293–303
Zhong X, Sun Y, Zhang Z, Dai Y, Wang Y, Liu Y, Hua R, Cao X, Liu Y (2019) A new hydrothermal cross-linking ion-imprinted chitosan for high-efficiency uranium removal. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-019-06794-1
Abd El-Magied MO, Hassan AM, Gad HMH, Mohammaden TF, Youssef MAM (2017) Removal of nickel (II) ions from aqueous solutions using modified activated carbon: a kinetic and equilibrium study. J Dispersion Sci Technol 39:862–873
Feng Y, He W, Zhang X, Jia X, Zhao H (2007) The preparation of nanoparticle Zirconiumium phosphate. Mater Lett 61:3258–3261
Misaelides P, Gallios G, Sarri S, Zamboulis D, Pavlidou E, Kantiranis N, Anousis I, Zhuravlev I, Strelko VV (2006) Separation of uranium from aqueous solutions using Al3+ and Fe3+ modified titanium- and zirconium phosphates. Sep Sci Technol 41:97–110
Zhou SM, Hu CB, Yuan L, Wei GJ, Jiao CX, Zhi Q, Jing B, Ping N (2013) Synthesis, characterization, thermodynamic and kinetic investigations on uranium(VI) adsorption using organic-inorganic composites: zirconiumyl-molybdopyrophosphate-tributyl phosphate. Sci China: Chem 56:1516–1524
Armento P, Casciola M, Pica M, Marmottini F, Palombari R, Ziarelli F (2004) Silica–zirconium phosphate–phosphoric acid composites: preparation, proton conductivity and use in gas sensors. Solid State Ionics 166:19–25
Marczenko Z, Balcerzak M (2000) Separation, preconcentration and spectrophotometry in inorganic analysis. Elsevier Science B.V, Amsterdam
Tag El-Din AF, Elshehy EA, Abd El-Magied MO, Atia AA, El-Khouly ME (2018) Decontamination of radioactive cesium ions using ordered mesoporous monetite. RSC Adv 8:19041–19050
Dong J, Ozaki Y (1997) FTIR and FT-Raman studies of partially miscible poly (methylmethacrylate)/poly (4-vinyl phenol) blend in solid states. Macromolecules 30:286–292
Tsezos M, Volesky B (1982) The mechanism of uranium biosorption by Rhizopus arrhizus. Biotechnol Bioeng 24:385–401
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1368
Elshehy EA, Shenashen MA, Abd El-Magied MO, El-Nahas AM, Tolan DA, Halada K, Atia AA, El-Safty SA (2017) Selective recovery of silver(I) ions from e-waste using cubicallymulti-thiolated cage mesoporous monoliths. Eur J Inorg Chem 2017:4823–4833
Freundlich H (1906) Adsorption in solution. Phys Chem Soc 40:1361–1368
Lagergren S (1898) About the theory of so called adsorption of soluble substance. Kungliga Svenska Vetenskapsakademiens Handlinger 24:1–39
Zhang X, Jiao C, Wang J, Liu Q, Li R, Yang P (2012) Removal of uranium(VI) from aqueous solutions by magnetic Schiff base: kinetic and thermodynamic investigation. Chem Eng J 198:412–419
Ding L, Deng HP, Wu C, Han X (2012) Affecting factors, equilibrium, kinetics and thermodynamics of bromide removal from aqueous solutions by MIEX composite. Chem Eng J 181:360–370
Bayramoglu G, Arica MY (2011) Preparation of a composite biosorbent using scenedesmus quadricauda biomass and alginate/polyvinyl alcohol for removal of Cu(II) and Cd(II) ions: isotherms, kinetics, and thermodynamic studies. Water Air Soil Pollut 221:391–403
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yousef, L.A., Bakry, A.R. & Abd El-Magied, M.O. Uranium(VI) recovery from its leach liquor using zirconium molybdophosphate composite: kinetic, equilibrium and thermodynamic studies. J Radioanal Nucl Chem 323, 549–556 (2020). https://doi.org/10.1007/s10967-019-06871-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06871-5