Abstract
The uranium sorption by anion exchange resins from productive solutions prepared by carbonate leaching from peat ore was investigated. The uranium recovery from leach liquors decreased during long-time exploitation of ion exchangers due to contamination of resins with humic substances. The alkaline solution of sodium chloride can be used to efficiently regenerate of resins. The resin Purolite A660/4759 had the highest value of full dynamic exchange capacity, which was (36.62 ± 1.10) g U/L of wet settled resin. The desorption process was carried out by ammonium carbonate/bicarbonate mixture solutions with values of uranium recovery degree of (97.41 ± 2.53)%. The degree of uranium precipitation during crystallization of ammonium uranyl carbonate from pregnant solutions was 72%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Uranium-containing peat deposits are generally considered as a source of environmental pollution but not as a source of raw material. Therefore, most of works presented in the literature are aimed at studying the migration of uranium in the surface layer, its association with humic substances, as well as identifying mechanisms and possible ways to prevent pollution of nearby rivers and reservoirs [1,2,3,4,5,6,7,8,9,10,11]. However, peat deposits can become objects of commercial use in the case of high uranium content in the ore and the present of possibility of ecosystem restoration during mining for further productive environmental management.
Technological schemes for the processing of peat ores usually include thermal destruction of enriched peat (burning or gasification), followed by sulfuric acid leaching of uranium from the ash, or direct leaching of uranium from the ore. The first method allows to additionally receive of electricity, heat and building sand, but requires high capital and operating costs [12]. Refusal from the energy-intensive operation of burning peat in favor of direct leaching of uranium from ore leads to a significant reduction in costs. Therefore, leaching method is currently the most promising. Wherein uranium can be extracted from peat ore using the method of percolation leaching (heap leaching or borehole leaching) in consideration of lack of need for expensive mining and shallow depth of ore location (from 0 to 8 m) [13,14,15,16].
Peat ores contain uranium in the oxidized form (UO22+). Uranium can be leached by use both acids and carbonate solutions as leaching agent. Acid leaching of uranium from peat is characterized by a high process rate. It is possible to use nitric, hydrochloric and sulfuric acid to carry out leaching process. However, the process of sulfuric acid leaching proceeds most effectively because of the formation of stable uranyl sulfate complexes [17,18,19,20]. The degree of uranium recovery from peat is not more than 75% when using sulfuric acid as a leaching agent. At the same time, there is a high consumption of sulfuric acid due to the interaction with organic substances and impurity elements. The process of carbonate leaching of uranium proceeds more slowly than acid leaching. However, the consumption of the reagent is lower, and the degree of uranium recovery from peat is more than 90%. Therefore, the leaching of uranium from peat ores using carbonate solutions is more economically viable. The disadvantage of this method is the effective dissolution of macromolecular humic substances (humic acid, fulvic acid) contained in peat. During sulfuric acid leaching, the organic content in the leach liquors is much lower and its value is approximately 50 mg L−1. The different degree of humic substances dissolution affects the color of solutions: liquors, obtained as result of acid leaching are colored in light yellow, and carbonate leach liquors are dark brown [8, 19, 21].
Ion exchange is the main method of processing of liquors obtained during leaching. Uranium exists in the form of [UO2(CO3)3]4− in carbonate solutions. The high value of the stability constant (2 × 1018) of this anionic complex excludes the presence of the cation UO22+ in the solution. Therefore, uranium recovery from leach liquors is possible only by use strongly basic anion exchangers, taking into account the alkaline character of medium [19, 20, 22]. The presence in leach liquor of humic substances leads to the poisoning of the ion exchanger and the fall of its capacity for uranium. Therefore, special attention is paid to the choice of ion exchange resin during designing of sorption plant for leach liquors processing.
The process of uranium sorption from productive sulphate and carbonate solutions using strongly basic anion exchangers has been studied in detail and presented in the literature [20,21,22,23,24,25,26]. However, all these works relate to uranium recovery from other types of ores, and there are no humic substances in productive solutions. The goal of works, devoted to uranium sorption from liquors obtained as result of carbonate leach of peat ores, is to clean up the area, because peat deposits are considered as a source of environmental pollution. In the course of presented study, we obtained results which are absent in the literature and which are of interest to researchers and the uranium industry. The novelty of our work is in the application of the ion exchange method for the uranium recovery from liquors of carbonate leaching of peat ores precisely with the production of uranium concentrate as a final commercial product, and not only for the purpose of environmental purification.
In this work, processes of uranium sorption from liquors, obtained during carbonate leaching from peat ore, using industrial anion exchangers AMP, Tulsion A-233U, Lewatit K6367, Purolite A660/4759 were studied. The main purpose of the work was to determine the capacitance characteristics of sorption resins for uranium and the degree of resin poisoning with humic substances during exploitation. The efficiency of uranium desorption from saturated anion exchangers using ammonium carbonate/bicarbonate mixture (ACBM) solutions with the subsequent crystallization of ammonium uranyl tricarbonate were determined.
Experimental
Anion exchangers Tulsion A-233U (Thermax Ltd, India), Lewatit K6367 (Lanxess Deutshland GmbH, Germany), AMP (SE Smoly, Ukraine) and Purolite A660/4759 (Purolite Ltd, United Kingdom) were used in this work. Specification of these resins from manufacturers is presented in Table 1. All anion exchangers were converted to carbonate form by contact with ammonium carbonate solution (150 g L−1) during 24 h. Sorption of uranium was carried out from liquors, which were obtained by carbonate leaching of uranium from peat ore of the Shu valley (near the border of Kazakhstan and Kyrgyzstan) [11]. The content of elements (compounds) in leach liquors was (mg L−1): 80 U, 3860 Na2CO3, 218 S, 286 Mg, 180 Si, 122 Al, 31 Ca. The concentration of humic substances (mainly in the form of sodium humate) was about 7000 mg L−1.
Uranium sorption was carried out in static and dynamic modes. The experiment in the static mode was led in a cylindrical closed vessel with volume 0.25 L at room temperature and constant stirring. The weight of the resin in air-dry state was 50 mg. A single load volume of leach liquors was 0.1 L. The phases contact time was 24 h. At the end of the experiment, the anion exchanger was separated from liquid phase by filtration, and solutions were analyzed for uranium content by ICP-AES. According to results of analysis, exchange capacity values of anion exchangers for uranium was calculated. After sorption and separation from solutions, the saturated anion exchange resin was transferred to a heat-resistant beaker and washed with water in several stages with constant stirring. Then, a two-stage desorption of uranium was carried out by use ACBM solutions as stripping agent. Sum concentration of ammonium carbonate and ammonium bicarbonate in these solutions were 100–200 g L−1. The volume of the stripping solution at each stage was 0.1 L. The process of uranium desorption was carried out with constant stirring and a temperature of 40–45 °C. The total desorption time was 4 h. After desorption, the anion exchanger was separated from pregnant solution and was washed with water in several stages with constant stirring. Then the resin was regenerated (washed from humic acids). The mixed solution 2% NaOH + 10% NaCl was used to remove absorbed humic substances from anion exchanger phase. The process of resin regeneration was carried out in a beaker for 4 h at room temperature and under constant stirring. Fresh portions of regenerating solution (volume 0.1 L) were filled into beaker every 1 h. After regeneration, the resin was washed with water, converted to the carbonate form and sent to the next stage of sorption. In this work, 10 cycles of “sorption–desorption—regeneration of the resin” were carried out. For comparison, 10-cycles series of “sorption–desorption” (without regeneration) experiments were led.
The sorption process in dynamic mode was carried out in a laboratory vertically located column. To determine yield curves of the sorption, 5 mL of the swollen anion exchanger was loaded into the column. Leach liquors were filtered continuously through a column at a constant rate of 0.025 L h−1 using a peristaltic pump. The filtrate (the solution leaving the column) was collected in fractions of 0.05–0.2 L and then was analyzed for uranium content by the ICP-AES method. The sorption process was finished after uranium concentrations equalizing in the solution at the inlet and outlet of the column.
The desorption process in a dynamic mode was carried out in a laboratory vertical column with a jacket using ACBM solutions (100–150 g L−1). A sample of saturated anion exchangers in a volume 5 mL was loaded into the column. The feeding of the eluents was carried out continuously with constant rate of 1 column volume for 1 h. The eluates fractions were collected, and then were analyzed for uranium content by the ICP-AES method. To ensure the maximum recovery of uranium, the elution process with ammonium carbonate/bicarbonate solutions was carried out in two stages at a temperature of 40–50 °C. A solution of ACBM with a concentration of 100 g L−1 was used at the first desorption stage. The ratio of the volume of liquid and solid phases in the first stage of desorption was 2:1. A concentration of ACBM in elution solution was 150 g L−1 at the second elution stage. Desorption was stopped when the concentration of uranium in pregnant solution decreased to a value less than 50 mg L−1. Pregnant solutions obtained during two stages of desorption were combined and cooled at room temperature. As a result, ammonium uranyl carbonate (AUC) crystals were formed. Pregnant solution was held for 12 h and then AUC crystals were separated by vacuum filtration. The uranium content in filtrate was determined by ICP-AES. The anion exchanger after desorption was washed with water and sent for regeneration (remove of humic acids).
After desorption, a sample of anion exchanger with a volume 4 mL (in the swollen state) was taken from the total volume of the resin. Then this sample was sent for regeneration. The regeneration process of anion exchangers in dynamic mode was carried out in a laboratory vertical column. The mixed solution 2% NaOH + 10% NaCl was used to remove humic substances from anion exchanger. The solution was fed to the column continuously at a constant rate of 4 mL h−1 (1 column volume for 1 h). Liquid and solid phases ratio during regeneration was 4:1. The total time of the process was 4 h. Samples of anion exchanger before and after regeneration were washed with distilled water, converted to carbonate form and used for sorption of uranium from leach liquors in static mode. Experiments were performed according to the method described above.
Optima 2100 DV (Perkin Elmer, USA) was used to determine the content of uranium in solutions. The automatic shaker IKA KS 3000 i control and magnetic stirrer with heating function IKA RET basic were applied to mix phases during sorption in static mode. The automatic LAMBDA OMNICOLL fraction collector, the IPC Ismatec peristaltic pump and thermostat Huber Compatible Control CC2 were used for investigation of the sorption/desorption/regeneration process in dynamic mode. All experiments were performed at least in duplicate, and the average error was less than 3%.
Results and discussion
Uranium sorption in static mode
According to the research results, the values of exchange capacity, obtained during uranium sorption from leach liquors in static mode, increased in the following sequence of ion exchangers: AMP → Tulsion A-233U → Lewatit К6367 → Purolite A660/4759. The capacity value of Purolite A660/4759 was (39.78 ± 0.80) g U/Lwsr (where Lwsr is liter of wet settled resin). Tulsion A-233U and Lewatit K6367 anion exchangers had similar capacity values: (28.63 ± 0.86) g U/Lwsr and (28.14 ± 0.70) g U/Lwsr, respectively. The exchange capacity of AMP resin was only (24.51 ± 0.56) g U/Lwsr.
The exchange capacity of anion exchangers during uranium sorption from solutions is influenced by many factors, and main factors are the initial concentration of uranium in solutions, the impurity composition of solutions and the pH value. There are no data necessary for an objective comparison on the capacitive characteristics of anion exchangers during the uranium sorption from solutions with a high content of humic substances in the literature. However, results obtained in this work are generally consistent with literature data on the uranium sorption from liquors of carbonate and sulfuric acid leaching [20, 22,23,24,25,26,27,28,29]. In works [25,26,27,28], uranium sorption isotherms from sulfate solutions are presented using a number of industrial grade anion exchangers. Exchange capacity values for anion exchangers AMP, Lewatit K6367 and Purolite A660/4759 is 28–32 g U/Lwsr, 20–23 g U/Lwsr and 44–46 g U/Lwsr, respectively, during uranium sorption from solutions containing 70–80 mg L−1 U in static mode. Exchange capacity values of strongly basic anion exchangers is about 30–45 g U/Lwsr during uranium sorption from carbonate solutions in static mode [20, 22,23,24]. Capacitive characteristics of anion exchangers obtained in this work are slightly lower than values presented above. There was a high content of humic substances in liquors obtained during the carbonate leaching of uranium from peat ore. Humic substances were recovered by anion exchangers together with uranium. This led to the poisoning of the resin, which results in a decrease of the sorption capacity values and a change in the color of the resin from yellow to brown or black.
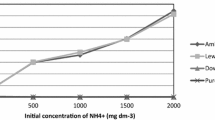
According to the results of research, under conditions of long-term exploitation of ion exchangers with an increase in the number of technological cycles, the value of exchange capacity for uranium decreased. Sorption capacity of anion exchangers for uranium after 10 cycles without resin regeneration was reduced on 29–30% (Fig. 1). At the same time, the fall in the exchange capacity under operating conditions, which include the resin regeneration stage (to remove of humic substances), during 10 cycles was no more than 5% (Fig. 2). This indicates the effectiveness of the use of an alkaline solution of sodium chloride for the resin regeneration. At the preliminary stage of the study, several reagent schemes were considered for the resins regeneration. In particular, HCl and NaOH solutions, hydrochloric-alkaline mixed solutions, sulfuric acid and nitric acid solutions with different concentrations were used for the regeneration of ion exchangers. As a result of preliminary experiments, it was found that the most effective is the alkaline solution of sodium chloride (2% NaOH + 10% NaCl). Therefore, solution with this composition was chosen to wash anion exchangers from humic substances in static and dynamic modes.
The effect of the number of exploitation cycles on exchange capacity value of resins during uranium sorption from leach liquors in static mode (without resin regeneration stage): 1—Purolite A660/4759; 2—Tulsion A-233U; 3—Lewatit К6367; 4—AMP. Conditions: mass of the resin (air-dry state) 50 mg; a single load volume of leach liquors was 0.1 L; contact time 24 h; constant stirring; room temperature)
The effect of the number of exploitation cycles on exchange capacity value of resins during uranium sorption from leach liquors in static mode (with resin regeneration stage): 1—Purolite A660/4759; 2—Tulsion A-233U; 3—Lewatit К6367; 4—AMP. Conditions: mass of the resin (air-dry state) 50 mg; a single load volume of leach liquors was 0.1 L; contact time 24 h; constant stirring; room temperature)
The use of sodium carbonate at the stage of uranium leaching from peat ore caused the presence of humic substances in leach liquors mainly in the form of sodium humate. The amount of fulvic acids and humic acids in leach liquors was insignificant. Large molecules of sodium humate occupied only the surface layers of the resin grains during sorption and did not hinder diffusion of uranium anionic complexes and humic acids into the resin phase. Sorption of humic acids was difficult due to the steric factor. However, fulvic acids were effectively attached to the matrix of resins and acted as carriers of functional groups (COOH). As a result of the sorption of humic substances, the color of anion exchangers was changed from yellow to dark brown or black. An analysis of industrial experience in the use of ionites in water treatment technology has shown that the presence of fulvic acids in anion exchangers reduces the internal diffusion coefficient of ions and increases the contribution of the stage of mass transfer inside resin grain. This leads to a deterioration in the kinetic properties of the anion exchange resin and, as a result, a decline in its exchange capacity. In this case, the resin must be regenerated more often. Then the volume of waste water will increase [8, 30,31,32,33,34,35].
Humic acids were washed out from poisoned resin phase and formed highly soluble sodium humate during the resin treatment with an alkaline solution of sodium chloride. Fulvic acids were retained by the anion exchanger and the process of the formation of fulvates proceeded extremely slowly. In addition, long-chain fragments of fulvic acids stayed on the surface of anion exchanger grains due to steric hindrance. Therefore, complete stripping of humic substances from anion exchanger phase was impossible in conditions of limited time for resin regeneration. This was indicated by the fact that resins were retained black or dark brown in the course of after regeneration study, i.e. the reverse color change of anion exchangers did not occured.
Uranium sorption in dynamic mode
According to the results of experiments carried out in static mode, the resin Purolite A660/4759 was chosen for studying the process of uranium sorption from leach liquors in dynamic mode because of best capacitive characteristics. Sorption curve of uranium by anion exchanger Purolite A660/4759 is shown at Fig. 3.
The results of the experiments shown a high efficiency of Purolite A660/4759 application for uranium sorption in dynamic mode from liquors obtained during the carbonate leaching of uranium from peat ore. Full saturation of resin was reached in 136 h of sorption (after pass of 680 bed volumes of productive solution through column), while the value of full dynamic exchange capacity was (36.62 ± 1.10) g U/Lwsr.
The Fig. 4 shown the initial part of the sorption curve. This area carries important information about the volume of the leach liquor that can be passed through the anion exchanger, so that the uranium content in the filtrate will satisfy the level of concentration in uranium waste. The content of uranium in filtrates at uranium industry enterprises is regulated at the level of 1 mg L−1. The value of dynamic exchange capacity for Purolite A660/4759 while achievement of uranium concentration 1 mg L−1 in filtrates in output of column was (13.50 ± 0.41) g U/Lwsr. The concentration level of 1 mg L−1 was achieved after pass of 170 bed volumes of leach liquors throuh resin.
Uranium desorption by ammonium carbonate/bicarbonate solutions
The value of full dynamic exchange capacity is one of important parameters, which influence on the choice of ion exchanger for sorption technology design. The degree of subsequent element desorption from phase of saturated resin is also important for this purpose. The main aim of ion exchange process is the maximum concentration of the metal in the minimum volume of pregnant solution. At the same time commercial concentrate should be easy obtained from the pregnant solution at minimal cost. A carbonate leaching method was chosen for uranium recovery from peat ore. That is why, it was decided to use ACBM solutions as eluent for uranium desorption of from anion exchangers.
The Purolite A660/4759 saturated anion exchange resin obtained as a result of uranium sorption from leach liquors in dynamic mode was used for this research. The value of full dynamic exchange capacity of this resin was (36.62 ± 1.10) g U/Lwsr. Results of investigation of uranium recovery from uranium-bearing peatlands by in situ leaching is presented in the work [12]. Leaching was performed with sodium carbonate. Sorption processing of productive solutions in a dynamic mode was carried out using anion-exchange resin AV-17-8. According to results of the study, the value of full dynamic exchange capacity of this anion exchanger was 42 g U/Lwsr. This capacity value is higher than that obtained in this work for Purolite A660/4759 due to the differences in composition of productive solution and the pH values. At present, in Russian uranium enterprises, values of full dynamic exchange capacity of anion exchangers (including AMP) used for the sorption processing of sulfate leach liquors, is 35–40 g U/Lwsr [26]. Full dynamic exchange capacity values of anion exchangers were about 40 g U/Lwsr during the sorption of uranium from carbonate solutions [24]. Consequently, using Purolite A660/4759 anion exchange resin for uranium recovery from leach liquors with a high content of humic substances will provide the high performance of sorption plant.
The choice of elution parameters was made on the basis of an analysis of the industrial experience in sorption technology exploitation for recovery and concentrating uranium by anion exchangers from in situ leach liquors. Such technology includes a desorption stage using solution of ACBM. The desorption process was carried out in two stages at a temperature of 40–50 °C to ensure maximum recovery of uranium from the resin. Increased temperature and a gradual increase of ACBM concentration in the elution solution makes it possible to avoid the precipitation of ammonium uranyl carbonate in the resin phase. Despite the fact that the solubility of AUC increases with temperature growth, it is not recommended to raise it above 60 °C, as this activates the process of decomposition of ammonium carbonate.
An ACBM solution with concentration of 100 g L−1 (on the sum of ammonium carbonate and ammonium bicarbonate) was used at the first stage of uranium desorption. Volume ratio of anion exchanger and eluent solution passed through column was 1:2. In the second stage, the desorption of uranium was carried out by solution with a sum concentration of 150 g L−1. The results of the research are presented in the Table 2 and Fig. 5.
Elution curve of uranium from saturated anion exchanger Purolite A660/4759 by ACBM solutions. Conditions: dynamic mode; vertically located column; the loaded volume of the swollen saturated resin—5 mL; eluent—ACBM solution (100–150 g L−1); the rate of eluent filtration—0.005 L h−1, temperature—40–50 °C)
According to the experimental data, the process of uranium desorption at each stage should be carried out at a ratio of the liquid and solid phase 2:1 (total—4:1). This makes it possible to extract (97.41 ± 2.53)% of uranium from saturated resin. The uranium concentration in the result pregnant solution was 8.92 g L−1, and the residual capacity of anion exchanger did not exceed (1 ± 0.03) g U/Lwsr. Pregnant solutions obtained during two stages of desorption were combined and cooled at room temperature. As a result, ammonium uranyl carbonate crystals were formed. Pregnant solution was held for 12 h. As an analysis results, the degree of uranium precipitation during crystallization of AUC was 72%, and the residual concentration of uranium in the mother solution was 2.5 g L−1.
The research results showed that about (79.87 ± 2.31)% of uranium was recovered from the resin in the first desorption stage and the uranium content in pregnant solution was 14.62 g L−1. At the second stage, (17.54 ± 0.51)% of the total amount of uranium was eluted, and concentration of uranium in pregnant solution was 3.21 g L−1. This concentration value was close to the metal content in mother liquor after AUC crystallization. Therefore, it is possible to eliminate the merging of streams of the two stages and use the pregnant solution after the second desorption stage to prepare the eluent solution.
Resin regeneration in dynamic mode by alkaline solution of sodium chloride
For studies of resin regeneration in a dynamic mode, the anion exchanger Purolite A660/4759, wich was acquired during the uranium sorption from productive solutions in column experiment, was used. The resin was taken after the stage of uranium desorption. A mixed solution 2% NaOH + 10% NaCl was used to remove the humic substances from anion exchanger phase. Purolite A660/4759 resin samples were washed with water before and after regeneration, then were converted to the carbonate form and used for uranium sorption from leach liquors in static mode. Capacitive characteristics of resin before and after regeneration were determined and were compared with respective values of fresh samples of anion exchanger (Table 3).
The research results confirmed the anion exchanger poisoning with humic substances during the uranium sorption from liquors of carbonate leaching, as well as the effectiveness of using an alkaline solution of sodium chloride for the regeneration of the resin. The decrease of the Purolite A660/4759 exchange capacity for uranium without regeneration and after this process was (14.81 ± 0.29)% and (4.97 ± 0.14)%, respectively. It was impossible to completely regenerate the resin during this study, therefore in the future it is planned to carry out experiments on the regeneration of the resin in accordance with the methodology, which provides for the alternation of the stages of long-term treatment by acid and by alkali solutions.
Conclusions
The sorption capacity values during the experiment of uranium sorption from leach liquors in static mode was increased in the following order of anion exchangers: AMP → Tulsion A-233U → Lewatit К6367 → Purolite A660/4759. The value of Purolite A660/4759 sorption capacity in static mode was about (39.78 ± 0.80) g U/Lwsr.
Under conditions of long-term exploitation of anion exchangers (10 cycles of “sorption–desorption” in static mode), the decrease of uranium capacity was about 30%, which was associated with poisoning of resin with humic substances. However, during the intermediate treatment of resins with an alkaline solution of sodium chloride (2% NaOH + 10% NaCl), the decline in the capacity of anion exchangers for uranium was no more than 5%. The use of this solution for the regeneration of resin was equally effective in organizing the process both in a static and dynamic modes. At the same time, it is not possible to completely regenerate the resin under the conditions of a limited time of the technological process because of poisoning with fulvic acids, the desorption of which from anion exchangers with gel structure is extremely difficult.
The full dynamic exchange capacity of the Purolite A660/4759 resin was (36.62 ± 1.10) g U/Lwsr. The high capacitive characteristics of Purolite A660/4759 anion exchanger allow to use this resin for the efficient uranium sorption from liquors obtained during the carbonate leaching of uranium from peat ore.
The use of ACBM solutions for uranium desorption from saturated anion exchangers ensured the production of pregnant solutions with a uranium concentration about 9 g L−1. The degree of uranium desorption was more than (97.41 ± 2.53)%, and the residual capacity of anion exchange resin did not exceed 1.1 g U/Lwsr. The degree of uranium precipitation during crystallization of AUC from pregnant solution was 72%, and the residual content of uranium in the mother liquors was 2.5 g L−1.
References
Bordelet G, Beaucaire C, Phrommavanh V, Descostes M (2018) Chemical reactivity of natural peat towards U and Ra. Chemosphere 202:651–660
Mikutta C, Langner P, Bargar JR, Kretzschmar R (2016) Tetra- and hexavalent uranium forms bidentate-mononuclear complexes with particulate organic matter in a naturally uranium-enriched Peatland. Environ Sci Technol 50:10465–10475
Cumberland SA, Douglas G, Grice K, Moreau JW (2016) Uranium mobility in organic matter-rich sediments: a review of geological and geochemical processes. Earth Sci Rev 159:160–185
Uralbekov BM, Smodis B, Burkitbayev M (2011) Uranium in natural waters sampled within former uranium mining sites in Kazakhstan and Kyrgyzstan. J Radioanal Nucl Chem 289:805–810
Arbuzov SI, Volostnov AV, Rikhvanov LP, Mezhibor AM, Ilenok SS (2011) Geochemistry of radioactive elements (U, Th) in coal and peat of northern Asia (Siberia, Russian Far East, Kazakhstan, and Mongolia). Int J Coal Geol 86:318–328
González AZI, Krachler M, Cheburkin AK, Shotyk W (2006) Spatial distribution of natural enrichments of arsenic, selenium, and uranium in a Minerotrophic Peatland, Gola di Lago, Canton Ticino, Switzerland. Environ Sci Technol 40:6568–6574
Read D, Bennett DG, Hooker PJ, Ivanovich M, Longworth G, Milodowski AE, Noy DJ (1993) The migration of uranium into peat-rich soils at Broubster, Caithness, Scotland, UK. J Contam Hydrol 13:291–308
Zielinski RA, Meier AL (1988) The association of uranium with organic matter in Holocene peat: an experimental leaching study. Appl Geochem 3:631–643
Owen DE, Otton JK (1995) Mountain wetlands: efficient uranium filters—potential impacts. Ecol Eng 5:77–93
Bordelet G, Beaucaire C, Phrommavanh V, Descostes M (2013) Sorption properties of peat for U(VI) and 226Ra in U mining areas. Procedia Earth Planet Sci 7:85–88
Matveyeva I, Jaćimović R, Planinšek P, Stegnar P, Smodiš B, Burkitbayev M (2014) Assessment of the main natural radionuclides, minor and trace elements in soils and sediments of the Shu valley (near the border of Kazakhstan and Kyrgyzstan). J Radioanal Nucl Chem 299:1399–1409
Evteeva LI, Sovenya NV, Khokhlov SV (2017) Experimental tests of uranium leaching from uranium-containing peat of the Kamyshanovskoye deposit. In: Presented at VIII international scientific and practical conference “actual problems of the uranium industry”, Astana
Edwards CR, Oliver AJ (2000) Uranium processing: a review of current methods and technology. JOM 52:12–20
Seredkin M, Zabolotsky A, Jeffress G (2016) In situ recovery, an alternative to conventional methods of mining: exploration, resource estimation, environmental issues, project evaluation and economics. Ore Geol Rev 79:500–514
Ilankoon IMSK, Tang Y, Ghorbani Y, Northey S, Yellishetty M, Deng X, McBride D (2018) The current state and future directions of percolation leaching in the Chinese mining industry: challenges and opportunities. Miner Eng 125:206–222
Ding D-x, Song J-b, Ye Y-j, Li G-y, Fu H-y, Hu N, Wang Y-d (2013) A kinetic model for heap leaching of uranium ore considering variation of model parameters with depth of heap. J Radioanal Nucl Chem 298:1477–1482
Razik AA, Ali FA, Attia FA (1989) Evaluation of the stability constants of uranyl association complexes with chloride, fluoride, bromide, and sulfate anions in solutions of constant ionic strength. Microchem J 39:258–264
Vopálka D, Štamberg K, Motl A, Drtinová B (2010) The study of the speciation of uranyl–sulphate complexes by UV–Vis absorption spectra decomposition. J Radioanal Nucl Chem 286:681–686
Morss LR, Edelstein NM, Fuger J (2010) The chemistry of the actinide and transactinide elements. Springer, Dordrecht
Rosenberg E, Pinson G, Tsosie R, Tutu H, Cukrowska E (2016) Uranium remediation by ion exchange and sorption methods: a critical review. Johns Matthey Technol Rev 60:59–77
Warwick P, Evans N, Hall A, Walker G, Steigleder E (2005) Stability constants of U(VI) and U(IV)-humic acid complexes. J Radioanal Nucl Chem 266:179–190
Song Y, Wang Y, Wang L, Song C, Yang Z, Zhao A (1999) Recovery of uranium from carbonate solutions using strongly basic anion exchanger: 4. Column operation and quantitative analysis. React Funct Polym 39:245–252
Nekrasova NA, Kudryavtseva SP, Milyutin VV, Chuveleva EA, Firsova LA, Gelis VM (2008) Sorption of uranium from carbonate solutions on various ion exchangers. Radiochemistry 50:180–182
Ladeira ACQ, Morais CA (2005) Uranium recovery from industrial effluent by ion exchange—column experiments. Miner Eng 18:1337–1340
Kolomiets DN, Troshkina ID, Sheremet’ev MF, Konopleva LV (2005) Sorption of uranium from sulfuric acid leaching solutions by strongly basic anion exchangers. Russ J Appl Chem 78:722–726
Skripchenko SY, Titova SM, Zhevlakova TA, Smirnov AL (2018) Uranium sorption from ISL solutions with an increased content of chlorides. In: AIP conference proceedings 2015, pp 020098
Rychkov VN, Smirnov AL, Gortsunova KR (2014) Sorption of uranium from underground leaching solutions with highly basic anion exchangers. Radiochemistry 56:38–42
Korovin V, Valiaiev O, Zontov O, Zontova L, Pilchyk V, Pysmennyi B (2019) Uranium (VI) sorption from sulphuric solutions by AM-p-2 anionite. In: E3S web of conferences 109, pp 00039
Sreenivas T, Rajan KC (2013) Studies on the separation of dissolved uranium from alkaline carbonate leach slurries by resin-in-pulp process. Sep Purif Technol 112:54–60
Fettig J (1999) Removal of humic substances by adsorption/ion exchange. Water Sci Technol 40:173–182
Shuang C, Wang J, Li H, Li A, Zhou Q (2015) Effect of the chemical structure of anion exchange resin on the adsorption of humic acid: behavior and mechanism. J Colloid Interface Sci 437:163–169
Levchuk I, Rueda Márquez JJ, Sillanpää M (2018) Removal of natural organic matter (NOM) from water by ion exchange—a review. Chemosphere 192:90–104
Bazri MM, Barbeau B, Mohseni M (2016) Evaluation of weak and strong basic anion exchange resins for NOM removal. J Environ Eng (United States) 142:04016044
Audenaert WTM, Van Beneden L, Van Hulle SWH (2016) Removal of natural organic matter (NOM) by ion exchange from surface water for drinking water production: a pilot-scale study. Desalination Water Treat 57:13897–13908
Grefte A, Dignum M, Cornelissen ER, Rietveld LC (2013) Natural organic matter removal by ion exchange at different positions in the drinking water treatment lane. Drink Water Eng Sci 6:1–10
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Skripchenko, S.Y., Titova, S.M., Smirnov, A.L. et al. Uranium sorption from productive solutions prepared by carbonate leaching from peat ore. J Radioanal Nucl Chem 322, 1825–1832 (2019). https://doi.org/10.1007/s10967-019-06751-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06751-y