Abstract
This study describes a rapid and novelty method for radiostrontium determination in aerosol filters and vegetation based on the use of plastic scintillation resins (PS resin), which combines the separation and measurement preparation into a single step. The optimization of the pre-treatment steps and the use of PS resin allows a simplification of the radiochemistry and a reduction in the time of analysis to 8 h and 12 h for aerosol filters and vegetation, respectively. The limits of detection were on average 0.04 Bq (filter)−1 and 3 Bq (kg-fresh)−1. The method obtained high recoveries (82% on average) and relative bias for total radiostrontium were below 30%. Individual activities of 89Sr and 90Sr were obtained by deconvolution methods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Radiostrontium isotopes (89Sr and 90Sr) are artificial radionuclides produced in nuclear reactors as fission products, but are also used in radioactive facilities as calibration sealed sources for different equipment (90Sr) and aqueous sources for bone cancer treatment in hospitals (89Sr). Accidents in these nuclear and radiological facilities or malevolent acts using radiological dispersal devices (RDDs) or “dirty bombs”, and improvised nuclear devices (INDs), can spread radiostrontium isotopes in the environment [1]. Atmospheric releases to the environment range from 0.04–13 PBq to 115 PBq for 89Sr, and 0.003–0.14 PBq to 10 PBq for 90Sr, in Fukushima and Chernobyl accidents respectively [2, 3].
Due to relatively long half-lifes, especially 90Sr (T1/2 = 28.79 years) and the high energy of the beta particles emitted (90Y, 2.28 MeV, from 90Sr, 0.55 MeV; and 89Sr, 1.50 MeV), they must be controlled from the point of view of radiological and environmental protection. In particular, population and animals can inhale radiostrontium present in aerosols, and animals can incorporate it by ingestion of the vegetation and migrate through the food-chain representing also an important risk.
In the environmental radiological surveillance programs in Spain (PVRA and REVIRA) supervised by the Spanish Nuclear Safety Council (CSN, in Spanish), radiostrontium is controlled in different environmental matrices (aerosol filters, water, vegetation, soil, milk and food). Moreover, the European Regulations limit their activity in food and feed following a nuclear accident or any other case of radiological emergency [4], and the US EPA established Analytical Action Levels (AALs), Analytical Decision Levels (ADLs) and Required Method Uncertainties (uMR) for their activities in water [5], air [1] and soil samples [6]. In all cases, the limits are more restrictive for 90Sr, due to its longer half-life and the additional contribution of 90Y.
Pure beta emitters, such as 89Sr and 90Sr, require a radiochemical separation prior to measurement and different methods for radiostrontium determination have been proposed in the literature [7, 8]. However, in case of emergency there is a need to develop rapid methods.
Different rapid methods for radiostrontium determination in aerosol filters and vegetation have been proposed in the literature [9,10,11,12,13,14,15]. A similar pre-treatment of the sample is performed, consisting on an acid digestion with HNO3, HCl, H2O2 and HF in an open vessel or microwave digester. In more complex samples microwave digestion is preferred to assure the complete dissolution of the sample [16], and in some methods a previous calcination of the sample is proposed to remove the organic matter and reduce the sample size [9, 11,12,13,14]. Moreover, apart from an open vessel digestion method [17], the US EPA proposed a fusion method with sodium carbonate for rapid dissolution of organic and glass-fiber filters [18]. Similarly, Maxwell et al. [10] dissolve vegetation samples by calcination and alkaline fusion with sodium hydroxide.
After dissolution, coprecipitation of strontium and calcium oxalates can be performed to concentrate alkaline earth elements, and some authors add Ca2+ carrier (20–200 mg) to improve strontium precipitation [9, 12]. Chobola et al. [11] proposed a Sr preconcentration in aerosol filters by cation exchange resins, and Maxwell et al. [14] carried out a lanthanum fluoride precipitation to eliminate part of the matrix interferents in the sequential separation of Sr and actinides in vegetation samples.
Regarding the separation step for isolation of strontium isotopes, currently the use of selective strontium chromatographic extraction resins is widespread [9,10,11,12, 14, 15]. Finally, gas flow proportional counting, scintillation counting or Cherenkov counting can be used for measurement.
The time of analysis is the most important factor to consider in case of emergency. Rapid methods found in the literature for radiostrontium determination in aerosol filters extend from 4 to 32 h, being the fastest method the one developed by Maxwell et al. [10]. On the other hand, radiostrontium determination in vegetation is more complex, with a duration that ranges from 8 to 72 h, being the fastest method the one developed by Maxwell et al. [14].
In addition, in case of the presence of 89Sr and 90Sr both peaks are going to be overlapped in the spectrum due to the continuous nature of the beta-ray spectra. For this reason, a separation technique needs to be applied for the individual quantification of each isotope in the less time possible. In the literature, there are different techniques for the separation of mixtures of beta spectra: window techniques, based on establishing different counting windows to determine the individual activity of each isotope [22]; multivariate calibration techniques, where partial least-squares (PLS) or principal component analysis (PCA) are used to develop models which can be used to predict the activities of unknown samples [23]; and spectrum deconvolution techniques, based on adjusting the shape of the individual spectra of the isotopes of interest to define mathematical functions and separate each signal [24,25,26].
In order to give a fast response in case of emergency, our approach is to optimize the pre-treatment steps for aerosol filters and vegetation samples, and to use plastic scintillation resins (PS resin) for strontium in the separation step. PS resins are based on plastic scintillator microspheres coated by a selective extractant, and unify chemical separation and sample measurement preparation in a single step [19, 20]. The main advantages are the reduction in time, effort and reagents required for analysis, because PS resin can be measured directly by scintillation counting. Moreover, unlike Liquid Scintillation (LS), no mixed waste is generated. The suitability of the PS resin for emergency situations was also demonstrated by the authors with the rapid method recently developed for radiostrontium determination in milk samples [21]. In addition, a deconvolution methodology is proposed, and the performance of three techniques, based on Fourier series, Legendre polynomials and Laguerre polynomials, to calculate the individual activities of 89Sr and 90Sr in a sample with a mixture of both were compared. Several types of aerosol filters and vegetation samples spiked with different proportions of 89Sr and 90Sr, and a reference material were employed to test and validate the method and the calibration system.
Experimental
Reagents and materials
All the reagents used were of analytical or reagent grade and standard solutions of 89Sr and 90Sr/90Y were supplied by Physikalisch-Technische Bundesanstalt (Braunschweig, Germany) and Amersham plc (Amersham, United Kingdom), respectively.
The plastic scintillation resins (PS resin) used in the study were produce by the Department of Chemical Engineering and Analytical Chemistry of the University of Barcelona. They are composed of plastic scintillation microspheres of 60 µm coated by a solution of DtBuCH18C6 in 1-octanol, in a proportion of 1:1/8 (PSm:extractant). For separation, 1.5 g of PS resin introduced in 2 mL solid-phase extraction cartridges (SPE cartridges) and a 12 position vacuum box from Triskem International (Rennes, France) were used. Both plastic scintillation microspheres and PS resin were prepared following procedures previously described by the authors [19,20,21]. The cartridges were measured within 20 mL polyethylene vials from PerkinElmer (Waltham, Massachusetts, USA).
Aerosol filters and vegetation samples
Nitrocellulose filters of 47 mm diameter and 0.8 µm pore size (MF-Millipore™) and Glass microfiber filters of 47 mm diameter and 1.2 µm retention (Filter-Lab) with an average residue of 10 mg in 1 week of air sampling were analysed. Different types of common vegetation in the Mediterranean forest were also considered. In particular, terrestrial vegetation (grass), bushes (rosemary) and aerial vegetation (pine needles).
An intercomparison sample of Spruce needles (Sample 04) in the IAEA-TEL-2016-03 worldwide open proficiency test was analysed to validate the method.
Equipment
A Quantulus 1220 (PerkinElmer) of the Universitat Politècnica de València was the scintillation spectrometer used in the study. Strontium analysis were performed on an AAnalyst 100/300 Atomic Absorption Spectrometer (PerkinElmer) in the laboratories of the Universitat Politècnica de València, and the elementary analysis was performed on an Optima 8300 ICP-OES spectrometer (PerkinElmer) in the laboratories of the University of Barcelona. A muffle furnace LKN 85 (Nannetti) was used for ashing the samples, and a microwave digestor Multiwave GO (Anton Paar) for dissolving the samples. A centrifuge Mixtasel BLT (J.P. Selecta) was used to centrifuge 50 mL polyethylene falcon tubes.
Procedure
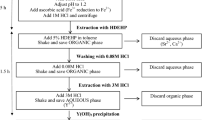
The procedures used to analyse aerosol filters and vegetation samples are composed of four steps: dissolution of the sample; concentration of strontium and alkaline-earth elements; separation by PS resin; and measurement in an scintillation counter. A complete scheme of the procedures are shown in Fig. 1.
Sample separation and measurement
In case of aerosol filters, a rapid calcination step is performed (30 min., 450 °C) in 250 mL porcelain crucibles with lid to destroy the cellulose filter and/or the organic matter present in the residue. Glass microfiber filters are not destroyed, but the elimination of carbon compounds improves the following dissolution. After calcination, the ashes are completely dissolved by microwave digestion following the steps shown in Table 1. Glass microfiber filters are cut into eight pieces before inserting them in the vessel. After step 2, the sample is evaporated to dryness to remove HF, and the residue is dissolved in diluted nitric acid and filtered in a 0.1 μm (pore size) in case some residue remains. The amount of 5 mg of strontium carrier (as Sr(NO3)2 solution) optimized in the previous study [21] is added in order to obtain the chemical yield in the separation, and 50 mg of Ca2+ carrier to improve the following oxalates co-precipitation due to the low amount of calcium in the aerosol filters. Some of the samples are also spiked with 90Sr/90Y and/or 89Sr tracers to test the method. The samples are then homogenized on a hot plate by stirring with a magnetic stirrer for 15 min. Oxalate precipitation is performed to separate strontium and other alkaline-earth metals from other groups. After heating, 2 g of oxalic acid are added to the solution and mixed for 5 min. The pH is then adjusted to 2–3 with ammonia, and the precipitate formed is centrifuged at 4200 rpm for 5 min and dissolved in 10 mL 6 M HNO3 (sample to pass through the PS resin) with the aid of a hot water bath to facilitate dissolution. An aliquot of the sample is taken to determine the chemical yield in the pre-treatment step by measuring strontium content by AAS or ICP-OES.
The pre-treatment steps for vegetation are similar, but in this case the calcination step is more complex. Routine methods perform a 24–48 h calcination step, but they can not be used in case of emergency where a fast response is needed. After testing different combinations of sample amount and temperature ramps, the selected conditions are: 5 g of fresh sample in each 250 mL porcelain crucible (a total of 10 g per sample) and two temperature ramps (25–250 °C, 1 h; 250 °C, 15 min.; 250–600 °C, 15 min.; 600 °C, 1 h) with a total duration of 2.5 h. Then, the ashes are dissolved by microwave digestion with the same protocol as the aerosol filters (Table 1). However, the ashes are split in three vessels (approximately 0.1 g in each vessel) to assure their complete dissolution. In case of vegetation, natural strontium content could be significant and it is determined by AAS or ICP-OES before adding the carriers to avoid systematic errors in the recovery. The oxalate precipitation is performed after adding Sr2+ and Ca2+ carriers, and 90Sr/90Y and/or 89Sr tracers in some samples. Then, the precipitate is dissolved in 10 mL 6 M HNO3 to pass through the PS resin.
After sample pre-treatment in both methods, strontium isotopes (89Sr and 90Sr) are isolated from other interferents present in the sample (mainly calcium) and 90Y (in case of 90Sr presence) by PS resin. The separation steps were optimized in the previous study [21], and consist of loading the sample after the PS resin conditioning with 2 mL 6 M HNO3. Then, the resin is rinsed with 2 mL 6 M HNO3 (2 times) and 2 mL 6 M LiNO3 (2 times) to remove the main interferents and avoid chemiluminiscence in the measurement due to the presence of nitric acid in the cartridge [20]. The flow rate is about 1 mL·min−1 in all cases. The load sample and all the rinses are collected in a 50 mL centrifuge tube and the strontium concentration is measured by AAS or ICP-OES. The difference between the strontium content in the sample solution and the waste gives the separation yield in the cartridge. Finally, the cartridge is placed in a 20 mL polyethylene vial and is directly measured in a Quantulus 1220 detector. Counting times were 60 min (3 cycles of 20 min) for the blank and the active samples and 10 min for the external standard gamma source to determine the Standard External Quenching Parameter (SQP(E)). In all cases, the cartridges were measured immediately after separation to avoid in growth of daughter radionuclides.
Regarding the time of analysis, the main differences between the methods for aerosol filters and vegetation are due to the time employed in the pre-treatment. The calcination step is longer in case of vegetation samples due to the higher organic content. Moreover, the volume in the evaporation step is three times higher than the volume in aerosol filters. For this reason, the aerosol filters pre-treatment lasts approximately 5.5 h, while for vegetation is 9.5 h. However, the time needed for PS resin separation is only 1 h, and for measurement and results calculation 1.5 h. Therefore, the time for radiostrontium separation in aerosol filters and vegetation is 8 h and 12 h, respectively.
Calibration standards
To determine the calibration efficiencies of the equipment, six standards with different activities of 89Sr (0.7 Bq to 29.5 Bq), six standards containing different activities of 90Sr (1 Bq to 31 Bq) and three blank samples were prepared. They were separated with PS resin and measured in the scintillation counter for 60 min. In all cases, the samples contained 5 mg of Sr2+ to obtain the recovery in the separation. Individual efficiencies for samples containing only one of the isotopes (89Sr or 90Sr) in the window (100–800) and combined detection efficiency for total radiostrontium as the mean of the individual efficiencies were calculated. In addition, six standards with different ratios of 89Sr:90Sr (1:1, 2:1, 5:1, 1:2, 1:5, 1:7) were prepared to verify calibration.
Data treatment and calculations
In case of a mixture of 89Sr and 90Sr, two options of calculations were employed: a system for total radiostrontium (89Sr + 90Sr) calculation, and a deconvolution system for 89Sr and 90Sr individual activity calculation.
Total radiostrontium activity (89Sr + 90Sr) in the samples was calculated by the following expression:
where cpm are the counts per minute of the sample or the blank, Eff is the combined efficiency of 89Sr and 90Sr or the individual efficiency when only one radionuclide is present in the sample, R1 is the pre-treatment recovery and R2 is the PS resin retention.
Pre-treatment recovery (R1) was calculated from the percentage ratio between the total amount of Sr2+ contained in the sample after adding the Sr2+ carrier and the amount of Sr2+ contained in the solution before being passed through the PS resin. In case of the vegetation, the natural Sr2+ content measured by ICP-OES or AAS has to be taken into account in the calculation; in aerosol filters the contribution is negligible. PS resin retention (R2) was calculated from the percentage ratio between the amount of Sr2+ contained in the PS resin (that is the difference between the amount of Sr2+ contained in the solution before being passed through the PS resin and the amount of Sr2+ contained in the wastes eluted from the SPE cartridge) and the amount of Sr2+ contained in the solution before being passed through the PS resin. Variance was obtained from the standard deviation of the different samples values.
For developing the deconvolution methodology, three different techniques were tested to analyse their performance in the spectral shape fitting: Fourier series [27], Legendre polynomials and Laguerre polynomials [28]. The deconvolution methodology is based on obtaining the spectral fitting of the individual calibration standards for different activities of 89Sr and 90Sr. As in emergency conditions the response must be as fast as possible, a measurement time of 1 h is considered, so the spectrum obtained contains a considerably amount of noise that has to be removed. Therefore, the spectrum of each radionuclide is smoothed using a Savitzky–Golay algorithm with an average window of 10 points for each side [29] and normalized (subtracting the spectrum of the equivalent blank solution and dividing the spectrum by the net count rate) to get an average normalized spectrum.
The activity of a certain sample is estimated assuming that the spectrum measured is given by the following expression:
where \(S_{mn}\) is the average spectrum reconstructed and \(C_{i }\) is a constant coefficient depending on the activity of the sample. Such coefficients are obtained by minimizing the error between the signal measured and the reconstructed spectrum using as an optimization technique the Nelder-Mead simplex direct search implemented in the “fminseach” function of Matlab [30]. Using those coefficients, the calibration curve of the activity versus the count rate for each radionuclide was fitted.
Finally, the spectrum of a mixture (89Sr and 90Sr) is fitted to a linear combination of the individual reconstructed spectra as:
Being a and b constants to be determined by the fitting process, by minimizing the error between the mixture spectrum and the linear combination of the individual spectra, using “fminsearch” in Matlab. The real activity corresponding to each one of the isotopes is computed using the calibration lines obtained and taking into account the total recovery in the separation. The window selected was 100–800.
The limit of detection (LD) was calculated with the blank samples following the equation proposed by Currie [31] and previously used [21].
Results and discussion
In the following section, the study of the separation steps for radiostrontium determinations in aerosol filters and vegetation in emergencies is presented. Regarding calibration, the results of 89Sr and 90Sr detection efficiencies are shown, and the development and validation of the deconvolution system is presented. Lastly, the method was tested and validated with different types of spiked samples and an intercomparison sample from the IAEA.
Sample separation study
After an exhaustive review of the literature, the separation steps for emergency radiostrontium analysis were selected trying to reduce the time of analysis. The main steps are a rapid calcination of the sample, microwave digestion, oxalate precipitation, PS resin separation and measurement by scintillation counting. Then, they were tested for different types of aerosol filters (glass microfiber and cellulose) and vegetation (grass, rosemary and pine needles). The results are described in the following sub-sections.
Influence of type of sample
Different types of aerosol filters and fresh vegetation samples were calcined following the optimized calcination steps explained before. In Fig. 2 are shown the samples before and after ashing. In glass microfiber filters only carbon compounds are decomposed, getting an average residue reduction of 60.2(4)%. Regarding cellulose filters, they are completely destroyed due to their organic composition. Vegetation samples remove their water content and organic matter.
The ashes were dissolved by microwave digestion and the solution was evaporated to dryness to remove HF. Then, the samples were filtered and the residue obtained was quantified. The percentage of digestion was 95.5 (5)% for glass microfiber filters, 98.3(1)% for cellulose filters, 93.1(10)% for grass, 96.3(5)% for rosemary and 87.0(10)% for pine needles. In addition, gross alpha and gross beta activity of the residue was determined by gas flow proportional counting, and the results were similar to background of the filter (0.1 cpm for alpha and 0.8 cpm for beta). From 4 to 9 replicates for each type of sample were digested.
Oxalate precipitation step and PS resin separation was performed in three replicates for each type of sample. Results of strontium recovery in the pre-treatment, the PS resin retention and the total recovery are shown in Table 2. Both, pre-treatment recoveries and PS resin separation are higher than 90% in almost all the samples and low deviation between replicates were obtained. The average total recoveries obtained range from 88 to 94% depending on the type of sample, which confirms that the complete procedure obtains good and reproducible recoveries for different types of aerosol filters and vegetation.
Elemental composition analysis
The elemental composition of the samples after microwave digestion was determined by ICP-OES. Table 3 shows the average results obtained of strontium and other elements for at least three replicates of each type of sample, and a range of values in case of vegetation due to the variability in composition depending on the water content of fresh samples.
Regarding natural strontium content, the amount in aerosol filters is negligible compared to the optimized amount of carrier added (5 mg Sr2+). However, the error can be higher than 5% (range 5–10%) in vegetation samples, thus it is necessary to determine the natural strontium content by ICP-OES or AAS in the sample before adding the carrier.
The main other elements in the samples are calcium, potassium, sodium, magnesium and phosphorus in case of vegetation, and additionally silicon in case of glass microfiber filters. Calcium content is very low in aerosol filters and very variable in the vegetation studied (from 5 to 71 mg). For these reasons, an amount of 50 mg of Ca2+ carrier was added in all types of samples to assure strontium coprecipitation with calcium oxalates.
Moreover, the behaviour of the interfering elements in the separation steps has been studied to prove the effectiveness of the proposed method. The average elemental composition of different aerosol filters and vegetation after oxalate precipitate dissolution and in the PS resin waste after separation were obtained. The results obtained were similar to the ones observed previously in milk samples [21]. The oxalate precipitation removes most of the interferents (K, Mg, Na and P) with average values between 95 and 100%. Calcium (76–89%), part of barium (10–28%) and sometimes part of magnesium (48%) precipitate with the oxalates, and they are removed in the following PS resin separation. Calcium retention in PS resin range from 10.7 to 29.3%, which correspond to an average of 17% of retention in comparison with the initial calcium content (natural + carrier added). Magnesium and barium retention is negligible in most of the samples. Therefore, the method achieves a high isolation of radiostrontium isotopes and permit their measurement by scintillation counting.
Calibration of 89Sr and 90Sr detection efficiency
Influence of sample matrix on detection efficiency
Several samples of aerosol filters and vegetation spiked with 89Sr or 90Sr were analysed with the proposed method and measured by scintillation counting to study the influence of the sample matrix on detection efficiencies. Figure 3 shows the same position of the spectra of 89Sr and 90Sr obtained for different types of aerosol filters (glass microfiber and cellulose) and vegetation (rosemary). For the optimum visualization of the shape of the spectra obtained, the spectrum acquired for each measurement was smoothed [29] and normalized.
Average detection efficiencies for 89Sr and 90Sr were 94(2)% (n = 3) and 90(1)% (n = 5) and the average quenching parameter (SQP(E)) was 676(7) (n = 10). For this reason, neither quenching effect nor matrix influence is observed in detection efficiencies.
89Sr and 90Sr detection efficiency calibration
Detection efficiencies were calculated with calibration standards with different activities of 89Sr and 90Sr and analysed using PS resin. The real spectra are shown in Fig. 4. The average quenching parameter was 680(12), and the average detection efficiencies obtained were similar to those obtained for the real samples: 93.3(3)% and 84.8(2)% for 89Sr and 90Sr, respectively. Combined detection efficiency of 89.1% was applied for total radiostrontium calculations when both isotopes are present in the sample.
The combined efficiency calculated was applied for six standards with different ratios of 89Sr/90Sr. The average quenching parameter was 678(8). The results for total radiostrontium activity (89Sr + 90Sr) calculated with the combined efficiency are shown in Table 4. Relative bias were in all samples below 5%, thus the approach can be used to calculate total radiostrontium.
Deconvolution method
Three deconvolution techniques were considered to estimate 89Sr and 90Sr activities: Fourier series, and Laguerre and Legendre polynomials. As an example, Fig. 5 shows the average normalized spectrum for 89Sr, the filtered spectrum and its reconstruction using Laguerre polynomials. It can be observed that the filter do not eliminate all the noise in the spectrum, and using a reconstruction technique the noise is completely removed.
Using Eq. (2) and after the optimization process, the calibration curves are obtained (Eq. 4):
Following the steps described above the three reconstruction techniques were used to estimate the activity of calibration standards spiked with different activities of 89Sr and 90Sr, shown in Tables 5 and 6. There are practically no differences between the activities estimated for each sample with all the methods and the relative bias of the estimation in comparison with the spiked activities are below 5% in all cases. For this reason, any method could be used to estimate 89Sr and 90Sr in PS resin, and in the following study only Legendre polynomials were employed because the results were slightly better in most of the samples studied.
The deconvolution method (Legendre polynomials) was applied to the samples spiked with different ratios of 89Sr/90Sr to estimate the individual activities of each radionuclide. As an example, Fig. 6 shows the spectra of samples spiked with ratios 1:1 and 1:2, and the deconvolution spectra of 89Sr and 90Sr. In addition, Table 7 shows the estimations and the relative bias obtained for each sample. Relative bias were below 30% in all the samples spiked with higher activities of 90Sr, but when 89Sr activities are higher, the reconstructions are overestimating 90Sr activity. However, 89Sr activities are correctly calculated, even they are acceptable when disproportion is 1:7. For these reasons, the deconvolution methodology proposed in this work can be considered as a useful tool to determine 89Sr and 90Sr activities in case of an emergency situation, except when there is a high 89Sr/90Sr disproportion and 90Sr is overestimated. An improved or alternative methodology is trying to be developed in this case.
Testing and validation of the method
The proposed method was applied to different samples of aerosol filters (glass microfiber filters and cellulose filters, with an aerosol residue of 1 week of sampling) and vegetation (10 g of grass, rosemary and pine needles). 14 samples spiked in the laboratory with certified standards of 89Sr and 90Sr in different ratios 89Sr/90Sr (1:1, 2:1, 4:1 and 8:1) were analysed to test the robustness of the method. As in a nuclear emergency, 89Sr was spiked always in higher proportion than 90Sr. An intercomparison sample of the International Atomic Energy Agency was also analysed to validate the method: 20 g of dry spruce needles (Sample 04) of the IAEA-TEL-2016-03, due to the low activity of the sample.
Average pre-treatment recoveries for the different types of samples were high, ranging from 76 to 97%, with an average of 87(10)%. PS resin retention was even higher, with an average value of 94(6)% that ranges from 87 to 99%, without great influence of the type of matrix. Finally, the average total recovery obtained for rapid strontium separation with the method proposed was 82(9)%, similar to the results obtained in the initial testing of the method (Table 2).
Total radiostrontium activities and individual activities of 89Sr and 90Sr were calculated for each sample. Combined detection efficiency of 89.1% was applied for total radiostrontium activity calculation of the spiked samples (M1 to M14), and individual efficiency of 84.8% was employed for 90Sr calculation of the intercomparison sample (M15), because any counts were observed in the 89Sr area. Deconvolution method was used for individual activity calculation of 89Sr and 90Sr. Table 8 shows the relative bias obtained for total radiostrontium activity (89Sr + 90Sr) and the results are below 20% in all types of aerosol filters and vegetation. The relative bias for the intercomparison sample was below 30% despite the low 90Sr activity (17 Bq (kg-dry)−1), which is the level of acceptance of the results. The average quenching parameter obtained for all the samples was 682(5). For these reasons, the method was validated for total radiostrontium activity. Moreover, 20 g of sample was analysed in this case, proving the robustness of the method for the analysis of higher amount of sample.
The relative bias for the individual activity of 89Sr and 90Sr were also calculated. As can be seen in Table 8, the results for 89Sr are good for all the samples, with values below 30%. In case of 90Sr, relative bias increase with higher ratios of 89Sr/90Sr. Most of the results are below 30%, except for samples M3, M11 and M14 with a ratio 8:1 were 90Sr activity is overestimated. Therefore, the deconvolution method is a useful tool to determine individual 89Sr and 90Sr activities in emergency situations, when the reduction of time is necessary, but we have to take into account that 90Sr activities are overestimated when there are high ratios 89Sr/90Sr. An improved or alternative methodology should be developed in this case.
Conclusions
A rapid and novel method has been developed to determine radiostrontium (89Sr and 90Sr) in aerosol filters and vegetation samples in emergencies. The method proposed performed a rapid pre-treatment of the sample and a separation of strontium isotopes by plastic scintillation resins (PS resin). PS resin unifies separation and measurement separation step achieving a reduction in the time of analysis and simplifying the method, which is necessary in case of emergency. Total radiostrontium activity was determined after calibration of the system of certified standards. Moreover, a deconvolution methodology is proposed and tested to determine individual activities of 89Sr and 90Sr, providing good results.
The method was validated with different types of aerosol filters (glass microfiber filters and cellulose filters) and vegetation samples (grass, rosemary and pine needles) spiked in the laboratory with different ratios of 89Sr/90Sr (1:1, 2:1, 4:1 and 8:1), and an intercomparison sample (spruce needles). Total recoveries obtained were high for all types of samples and quite reproducible, with an average of 82(9)%. Relative bias for total radiostrontium were also below 20% for the spiked samples and below 30% for the intercomparison sample. Relative bias for individual activities 89Sr and 90Sr were below 30% in almost all the samples, but 90Sr activity is overestimated when ratios 89Sr/90Sr are high.
The time of analysis of the proposed methods for radiostrontium determination in aerosol filters and vegetation is 8 and 12 h, respectively, which are less than a day and a half working day. Limits of detection in 1 h of counting for aerosol filters are between 0.03–0.05 Bq (filter)−1 and 2.8–3.7 Bq (kg-fresh)−1, for 10 g of fresh vegetation samples. Therefore, the proposed methods are suitable to be applied in emergency situations.
References
EPA (2009) EPA 402-R-09-007, radiological laboratory sample analysis guide for incidents of national significance—radionuclides in air. U.S. Environmental Protection Agency, Montgomery
International Atomic Energy Agency (2015) The Fukushima Daiichi accident, technical volume 4, radiological consequences. IAEA, Vienna
International Atomic Energy Agency (2006) Environmental consequences of the chernobyl accident and their remediation: twenty Years of experience, report of the UN chernobyl forum expert group “Environment”, Radiological assessment reports series no. 8, IAEA, Vienna
Council Regulation (Euratom) 2016/52 of 15 January 2016 laying down maximum permitted levels of radioactive contamination of food and feed following a nuclear accident or any other case of radiological emergency, and repealing Regulation (Euratom) No 3954/87 and Commission Regulations (Euratom) No 944/89 and (Euratom) No 770/90
EPA (2008) EPA 402-R-07-007, radiological laboratory sample analysis guide for incidents of national significance—radionuclides in water. U.S. Environmental Protection Agency, Montgomery
EPA (2012) EPA 402-R-12-006, radiological laboratory sample analysis guide for incident response—radionuclides in soil. U.S. Environmental Protection Agency, Montgomery
Vajda N, Kim CK (2010) Determination of radiostrontium isotopes: a review of analytical methodology. Appl Radiat Isot 68:2306–2326
Shao Y, Yang G, Tazoe H, Ma L, Yamada M, Xua D (2018) A review of measurement methodologies and their applications to environmental 90Sr. J Environ Radioact 192:321–333
Wang J (2013) A quick liquid scintillation counting technique for analysis of 90Sr in environmental samples. Appl Radiat Isot 81:169–174
Maxwell SL, Culligan B, Noyes G (2010) Rapid separation method for actinides in emergency air filter samples. Appl Radiat Isot 68:2125–2131
Chobola R, Mell P, Daróczi L, Vincze A (2006) Rapid determination of radiostrontium isotopes in samples of NPP origin. J Radioanal Nucl Chem 267(2):297–304
IAEA (1999) IAEA-TECDOC-1092, Generic procedures for monitoring in a nuclear or radiological emergency. International Atomic Energy Agency, Viena
Amano H, Sakamoto H, Shiga N, Suzuki K (2016) Method for rapid screening analysis of Sr-90 in edible plant samples collected near Fukushima, Japan. Appl Radiat Isot 112:131–135
Maxwell S, Culligan B, Noyes G (2010) Rapid separation of actinides and radiostrontium in vegetation samples. J Radioanal Nucl Chem 286:273–282
Martin J, Odell K (1998) The development of emergency radioanalytical techniques for the determination of radiostrontium and transuranic radioisotopes in environmental materials. Radioact Radiochem 9(3):49–59
Janda J, Sladek P, Sas D (2012) Rapid dissolution of biological samples using microwave digestion for determination of radionuclide contamination by liquid scintillation. J Radioanal Nucl Chem 293:223–229
EPA (2012) EPA 402-R12-009, rapid method for acid digestion of glass-fiber and organic/polymeric composition filters and swipes prior to isotopic U, Pu, Am, Sr and Ra analyses for environmental remediation following homeland security events. U.S. Environmental Protection Agency, Montgomery
EPA (2012) EPA 402-R12-008, rapid method for sodium carbonate fusion of glass-fiber and organic/polymeric composition filters and swipes prior to isotopic U, Pu, Am, Sr, Ra analyses for environmental remediation following homeland security events. U.S. Environmental Protection Agency, Montgomery
Tarancón A, Bagán H, García JF (2017) Plastic scintillators and related analytical procedures for radionuclide analysis. J Radioanal Nucl Chem 314:555–572
Bagán H, Tarancón A, Rauret G, García JF (2011) Radiostrontium separation and measurement in a single step using plastic scintillators plus selective extractants. Application to aqueous sample analysis. Anal Chim Acta 686:50–56
Sáez-Muñoz M, Bagán H, Tarancón A, García JF, Ortiz J, Martorell S (2018) Rapid method for radiostrontium determination in milk in emergency situations using PS resin. J Radioanal Nucl Chem 315:543–555
Kim CK, Al-Hamwi A, Toervenyi A, Kis-Benedek G, Sansone U (2009) Validation of rapid method for the determination of radiostrontium in milk. Appl Radiat Isot 67:786–793
Fons-Castells J, Tent-Petrus J, Llauradó M (2017) Simultaneous determination of specific alpha and beta emitters by LSC-PLS in water samples. J Environ Radioact 166:195–201
Grau Malonda A, Rodriguez Barquero L, Grau Carles A (1994) Radioactivity determination of 90Y, 90Sr and 89Sr mixtures by spectral deconvolution. Nucl Instrum Meth A 339:31–37
Altzitzoglou T (2008) Radioactivity determination of individual radionuclides in a mixture by liquid scintillation spectra deconvolution. Appl Radiat Isot 66(6–7):1055–1061
Remetti R, Sessa A (2011) Beta spectra deconvolution for liquid scintillation counting. J Radioanal Nucl Chem 287(1):107–111
Grau Carles A, Grau Malonda A (1990) Spectral Interpolation and unfolding to measure multi-labelled samples by liquid scintillation. Ciemat 675, Sp lSSN 614-087-X
Eisinberg A, Fedele G (2007) Discrete orthogonal polynomials on equidistant nodes. Proc Int Math Forum 21(2):1007–1021
Savitzky A, Golay MJE (1964) Smoothing and differentiation of data by simplified least-squares procedures. Anal Chem 36:1627–1639
Nelder JA, Mead R (1965) A simplex method for function minimization. Comput J 7(4):308–313
Currie LA (1968) Limits for qualitative detection and quantitative determination. Application to radiochemistry. Anal Chem 40(3):586–593
Acknowledgements
The authors are grateful to the Spanish Ministerio de Economia y Competitividad (MINECO) for financial support, under CTM2017-87107-R and the Catalan Agència de Gestió d’Ajuts Universitaris i de Recerca (AGAUR) for financial support, under 2017-SGR-907. We should also like to thank the Universitat Politècnica de València for finantial support under the “Programa propio para la Formación de Personal Investigador (FPI) de la Universitat Politècnica de València—Subprograma 1”, and the Valencian Agency for Security and Emergency for their support (S7042000 (2018)) in the “Development of the Emergency Radiological Surveillance Plan” in the Valencian Community (Spain).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sáez-Muñoz, M., Bagán, H., Tarancón, A. et al. Rapid methods for radiostrontium determination in aerosol filters and vegetation in emergency situations using PS resin. J Radioanal Nucl Chem 322, 1397–1408 (2019). https://doi.org/10.1007/s10967-019-06779-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06779-0