Abstract
The present work dealt with selective leaching of uranium from Alloga carbonaceous shale which assays 0.136% of U associated to 0.177% Cu and 0.068% of rare earth elements, based on an environmentally friendly method, using Na2CO3/NaHCO3 solution. The carbonate leaching kinetics was studied to determine the nature of the dissolution process. Applying the un-reacted shrinking-core model in the solid–liquid phase reactions, it can be inferred that the predominant dissolution mechanism of uranium is diffusion controlled only. The apparent activation energy (Ea) was estimated to be 9.320 kJ/mol. The study focus was then shifted to the recovery of 99.7 U using anion exchange Amberlite IRA400 resin at pH 8.5 and about 93% of the adsorbed U were regenerated using 10% sodium bicarbonate solution. Finally, the regenerated U-rich solution was treated with H2O2 solution to precipitate UO4∙2H2O which achieved a precipitation efficiency of 99%. The latter was carefully washed and ignited at 850 °C to prepare pure U3O8.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
An interesting rare metal mineralization was recorded in Alloga locality of Abu Zienema area. This mineralization is mainly associated with different rock facies including shale, siltstone, clay, ferruginous sandstone, calcareous sandstone and feldspathic sandstone (EL Assy et al. 1986; Abdel Monem et al. 1997). Alloga carbonaceous shale facies of Um Bogma Formation is considered as one of the most important occurrences of U mineralization beside the associated economic metal values, e.g., REEs, B, V, Co, Ni, Zn, Cu, etc. (Al Shami 2003; Abdellah 2014; Abu Khoziem 2017).
Uranium is generally leached by two principal methods namely acidic or alkaline method. The choice of either depends mainly upon the overall composition of the ore and its effect upon the reagent consumption. Alkali carbonate (sodium or less commonly ammonium) is sometimes used for uranium leaching from its ores of high in carbonate minerals like calcite, dolomite, etc. (Suri et al. 2009; Santos and Ladeira 2011). This depends on the fact that the carbonate anion forms stable soluble uranyl carbonate complex [UO2 (CO3)N2 − 2n. It can be applied to both primary and secondary mineral deposits, however, after oxidation of the former (Lunt et al. 2007). Alkaline leaching is generally characterized by producing a comparatively pure solution due to its relative selectivity, easy regeneration and recycling beside minor corrosion problems (Abhilash and Pandey 2013; El Ansary et al. 2017).
El-Sheikh et al. (2015) studied in detail the selective recovery of U and Cu from carbonate-rich latosol ore material occurring at Abu Thor locality of southwestern Sinai mineralization by applying two successive alkaline leaching processes. Selective uranium leaching was performed using urea, while copper was subsequently and relatively leached with mixed solutions of ammonium hydroxide and ammonium carbonate. Abu Khoziem (2017) studied the mineralogy and recovery of U from Alloga carbonaceous shale. Different leaching studies have been investigated including alkali agitation leaching, H2SO4 acid agitation leaching and pug leaching method. Also, the alkali agitation alkali agitation leaching using Na2CO3 is not efficient in dissolving both U and other associated. The latter was found to be preferred under the following conditions; 1.35 ton H2SO4/ton ore at 110 °C for 2 h.
In this context, Oraby et al. (2018) studied the Cu/U mineralization and suggested alakline leaching for the recovery its metal values. The relevant factors of alkaline leaching of a technological sample were studied using a mixture of 150 g Na2CO3/(NH4) HCO3 solution. Under the most favorable conditions of contact time of 180 min. at 80 °C and S/L of 1/5 the leaching process efficient to dissolve about 93.75% U and 97% Cu. Recovery of the leached metal values was performed using ion exchange for the former and direct precipitation for the latter.
In the alkaline medium, uranium is capable of forming anionic species mainly as uranyl carbonate complexes, [UO2 (CO3)2]2− and [UO2 (CO3)3]4− (Li et al. 2006; Hunter 2013). The anion exchanger is the most suitable resin for uranium, when it is mainly present as uranyl carbonate complexes. Anion exchange resins have been successfully employed to recover uranium from its leach liquors (Seneda et al. 2001; Nascimento et al. 2004; Ladeira and Morais (2005); Santos and Ladeira 2011; Muhammad et al. 2017). Finally, the aims of the present work is characterize (explain) the Alloga carbonaceous shale either chemically, mineralogically with studying the kinetics of carbonate leaching process. The purpose was to prepare U cake with the possible purity. To achieve this goal, a technological sample was properly collected representing the studied carbonaceous shale.
Experimental
Characterization of the ore material
To achieve the suitable leaching method for the working ore material, it was decided to study its chemical and mineralogical composition. Complete chemical analysis was investigated including the major elements oxide beside the associated trace elements. For this purpose, a representative sample portion of the collected technological sample was properly prepared. The major elements oxide was analyzed following the method given by Shapiro and Barnnock (1962). With respect to Ca and Mg, a titrimetric method with EDTA was performed. The spectrometric analysis was applied for estimation of SiO2, Al2O3, TiO2 and Fe2O3 (total); while for Na2O and K2O, the flame photometry was used. Weighted sample portions were used to estimate that the loss of ignition (obtained at 1000 °C) is corresponding to humidity, combined water, CO2 as well as possible organic matter. On the other hand, a visible–ultra-violet spectrometer was used for the quantitative analysis of total REEs using 0.05% arsenazo III at λ 654 nm using Ce as reference (Marczenko 2000). An atomic absorption spectrometer was used for analysis of heavy metals, e.g., Cu, Co, Ni, Mn, etc. at the proper wave lengths. For U determination, the oxidometric titration method was applied against NH4VO3 (Mathew et al. 2009).
To investigate the mineralogical composition of the study sample, the heavy mineral separation procedures were applied. The whole disaggregated sample was then deslimed by washing and decantation. After drying, the sample was properly sieved using a set of sieves ranging from − 30 to + 120 meshes (595 to 125 µ) and the obtained size fractions were subjected to heavy liquid separation using bromoform (sp. gr. 2.84). The obtained heavy mineral fractions were investigated using binocular microscope where some picked mineral grains were identified by X-ray diffraction technique (XRD).
Optimization of leaching and extraction procedures
Several experiments were performed to optimize the agitation leaching of 10 gm from the ground sample with different Na2CO3/NaHCO3 concentrations at different solid/liquid ratios and stirring for different periods of time at different temperatures. The leached U was estimated in all agitation leaching streams to calculate its leaching efficiency. After the detection of the leaching optimum conditions, a sample weight of 250 g was used for the preparation of the pregnant alkaline leach liquor required for U extraction process. In this context, the anion exchange resin Amberlite IRA400 in its carbonate form was used for optimization of uranium extraction process from its carbonate leach liquor. For this purpose, batch experiments were conducted using different volumes ratios of wet settled resin (WSR) and leach liquor (R/L) at different pH values and different stirring times. The raffinate solutions were analyzed for uranium and its extraction efficiency was calculated. The loaded resin, after washing with distilled water, was subjected to the elution process to regenerate the loaded uranium using 10% NaHCO3 solution. The eluate-rich uranium solution was then subjected to U precipitation using H2O2.
Results and discussion
Characteristics of the study carbonaceous shale
Chemical composition
From the obtained data of the major constituents of the working sample, Table 1, it is clearly evident that the studied sample is mainly composed of high concentration of carbonates (23.5% L.O.I., 8.15% of CaO and 6.35% of MgO) besides SiO2 (45.20%), Al2O3 (7.10%) and moderate iron oxide (6.75%). These oxides are chiefly allotted as calcium carbonate and magnesium-silicate minerals. It is worthy to mention herein that the organic matters together with the carbonate minerals represent about 38% of the total chemical composition of the studied sample; this matter reflects the carbonaceous nature of the studied sample. With respect to the valuable metal elements, it was found that U, Cu and REEs assayed to 0.136%, 0.177% and 0.068%, respectively, which reflects the significant high grade of mineralization. From chemical composition of the studied technological sample, it can be concluded that it might be in favor of applying an alkali leaching procedure.
Mineralogical composition
For heavy liquid separation, the size fraction ranged from − 30 to + 120 meshes (595 to 125 µ) was deslimed followed by drying at 110 °C, and then subjected to heavy liquid separation for upgrading the heavy fractions before mineralogical analysis. Investigation of the obtained heavy mineral fractions of sample under the binocular microscope revealed that dolomite and gypsum are the main mineral constituent stogether with the gangue constituents, e.g., goethite, hematite and quartz.
Some of the picked mineral grains from the upgraded heavy fractions were investigated by X-ray diffraction analysis. Unfortunately, no economic mineral species corresponding to U and/or rare earth elements has been identified. This can be interpreted as U and RE elements not forming specific discrete mineral but rather incorporated within organic matter which is present in high ratio in the study sample (Jaireth et al. 2008). On the other hand, rare earth elements and uranium element may exist as adsorbed ions on the iron oxy-hydroxides and carbonate minerals (Kuşcu et al. 2016; Ali 2016) which incorporated within oragnic matter which is present in high ratio in the study sample.
It is worthy to mention herein that the present mineralogical study revealed the presences of two main types of Cu mineralization. The first type was of the alteration (oxidation) zone such as atacamite CuCl(OH)3 and malachite Cu CO3 (OH) (Table 2). While the second type was of the copper sulfide ones (reduction zone) such as chalcopyrite CuFeS2 (Table 2). Finally, from the above mineralogical study, it can be concluded that, this mineral assemblage together with the gangue constituents reflects the non-refractory nature of the study material.
Optimization of alkaline agitation leaching
Both chemical and mineralogical data reflect that the studied technological sample is mainly carbonaceous shale. Thus, the alkali leaching method was preferred compared to acidic one. The following leaching parameters were studied to optimize alkaline leaching conditions:
Effect of Na2CO3/NaHCO3 concentration
This factor was studied using different Na2CO3/NaHCO3 mixture solution concentrations ranging from 5 to 20%. The other leaching conditions were fixed at room temperature (35 ± 5 °C) for 2 h with S/L ratio of 1/2 and 3/1 Na2CO3/NaHCO3 mixed ratio. The obtained data (Fig. 1a) showed that when Na2CO3/NaHCO3 concentration increased from 5 to 10%, U leaching efficiency increases from 33.1 to 63.2%. Any further increase of the alkali mixture concentration up to 20%, the U leaching efficiency decreases to 33.8%. This mean that, by increasing the alkali concentration no increasing in leaching efficiency occur.
a Effect of Na2CO3/NaHCO3 mixture solution concentration upon U leaching efficiency at room temperature for 2 h with S/L ratio of 1/2 and 3/1 Na2CO3/NaHCO3 mixed ratio. b Effect of S/L ratio upon U leaching efficiency at room temperature (35 ± 5 °C) for 2 h, 10% Na2CO3/NaHCO3 and 3/1 Na2CO3/NaHCO3 mixed ratio. c Effect of Na2CO3/NaHCO3 ratio upon U dissolution efficiency at S/L ratio of 1/2 with stirring time for 2 h at room temperature. d Effect of temperature upon U leaching efficiency at Na2CO3/NaHCO3 of 10%, S/L ratio of 1/2 and 2 h. e Effect of time upon U dissolution efficiencies at Na2CO3/NaHCO3 of 10%, S/L ratio of 1/2 and leaching time of 90 °C
Effect of solid/liquid ratio
Different solid/liquid ratios (S/L) ranging from 1/2 to 1/5 were studied to detect the effect of S/L ratio upon U leaching efficiency using 10% Na2CO3/NaHCO3 for 2 h at room temperature. The obtained data (Fig. 1b) indicated that U leaching efficiency decreased from 63.2% up to 38.7% by increasing S/L ratio from 1/2 up to 1/5. This may be attributed to the dissolution of some interfering elements.
Effect of Na2CO3/NaHCO3 ratio
This factor was studied using 10% Na2CO3/NaHCO3 in different carbonate mixed ratios ranging from 1:1 to 4:1 Na2CO3/NaHCO3. The other leaching conditions were kept constant using S/L ratio of 1/2 with stirring time for 2 h at room temperature. Data shown in (Fig. 1c) clarified that the Na2CO3/NaHCO3 mixed ratio of 3/1 is more effective upon U leaching efficiency compared to the other mixed ratios.
Effect of leaching temperature
The remarkable influence of different leaching temperatures ranging from 35 °C up to 95 °C upon U leaching efficiency was conducted in Fig. 1d. The other leaching conditions were kept constant at Na2CO3/NaHCO3 of 10%, S/L ratio of 1/2 and leaching time of 2 h. From the obtained data, it is clearly evident that the U leaching efficiency significantly improved from 63.2% to its maximum value (99.3%) with increasing leaching temperature from 35 °C up to 95 °C.
Effect of leaching time
This factor was already investigated at the obtained optimum leaching condition changing the leaching time periods from 0.5 to 3 h. The corresponding leaching efficiency (Fig. 1e) indicated that the leaching time of 2 h is very suitable to dissolve 99.3% of U.
Finally, from the foregoing alkaline agitation leaching study, it can be concluded that this technique is more efficient in selective leaching of 99.3% U from the studied carbonaceous shale sample at the optimum leaching conditions summarized as:
Na2CO3/NaHCO3 ratio: 3/1
Na2CO3 + NaHCO3 concentration: 10%
Solid/Liquid ratio: 1/2
Leaching temperature: 90 °C
Leaching time: 2 h
Kinetics of carbonate leaching Study
The effect of leaching time upon the dissolution of U at different temperatures was carried out at optimum leaching conditions of − 200 mesh (74 µ) particle size, 10% Na2CO3 + NaHCO3, solid/liquid ratio 1/2 at temperature range between 35 and 90 °C. Figure 2 shows that the leachability of uranium increases gradually by increasing time and temperature. The maximum leachability was found to be 99.3% at 90 °C and after leaching time of 2 h.
Application of leaching kinetic models
The un-reacted shrinking-core model is the most commonly used mathematical model to describe the heterogeneous reactions like mineral leaching from its ores. In the solid–liquid phase reactions, the rate of reaction is controlled by the following steps: liquid-film diffusion (mass transfer), solid or product layer diffusion, and surface reaction or chemical reaction. One or more of these factors might control the rate of the reaction (Levenspiel 1999). Amongst the three controlling mechanism, the liquid-film diffusion resistance is eliminated or minimized by effective stirring.
To determine the type of leaching mechanism prevalent for the uranium, some reaction models were investigated to find which kinetic equation can fit the reaction isotherms. The results were analyzed using the following kinetic rate Eqs.
Reaction rate expression controlled by the surface chemical reaction:
where Kc is the rate constant (min−1) for chemical reaction.
Reaction rate expression controlled by the diffusion through the ash or product layer:
where Kd is the rate constant (min−1) for diffusion through the product layer.
Figure 3a and b shows the result of plotting 1 − (1 − x)1/3 and 1 − 3(1 − x)2/3 + 2(1 − x) as a function of time at different leaching temperatures. The values of the reaction rate constants K were determined from the slope of the straight line of the relation between kinetic model and time.
The Kc and Kd values computed from Eqs. (1) and (2) are given in Table 3. The R2 values mean the extent of fitting between the experimental data and the predicted one. The best fit has R2 of nearly 1.0. The Kc values given in Table 3 vary in the rage of 0.0016–0.003 min−1, while the Kd were between 0.0023 and 0.0042 min−1. The R2 values for Kd were 0.989–0.95; while for Kc, it was in the range of 0.86–0.96. Based on the R2 values, it can be inferred that the predominant dissolution mechanism of U from the Abu Zienema carbonaceous shale ore is diffusion controlled only.
Calculation of the activation energy
The logarithmic values of these reactions rate constants Kd were plotted against the reciprocal of the absolute leaching temperature according to the Arrhenius equation shown in Fig. 4.
The activation energy of the reaction can be calculated using the following equation:
where k is a reaction rate constant, recovery (conversion fraction) in min−1. A is the frequency factor, constant min−1. Ea is the apparent activation energy kJ mol−1. Rg is the universal gas constant = 8.314 JK−1 mol−1. T is the reaction temperature K.
From Fig. 4, the activation energy (Ea) was calculated as follows: \({\text{Slope}} = \frac{{ - E_{\text{a}} }}{Rg}\)
For expression the reaction rate equation,
The apparent activation energy (Ea) was calculated from the slope of straight line obtained to be 9.320 kJ/mol for diffusion-controlled reaction models. Based on the (Ea) values, it can be inferred that the predominant dissolution mechanism of U from Alloga carbonaceous shale is diffusion controlled only. This value is less than the amount mentioned by Crundwell (2013) who pointed out that the activation energy for diffusion-controlled reactions is below 20 kJ/mol and it is above 40 kJ/mol for chemical-controlled reactions.
Results of the uranium extraction
Applying the above-mentioned optimum leaching conditions upon 250 g of Alloga carbonaceous sample yields 1 L of carbonate solution of pH 10 and assaying 0.34 g/L of U as given in Table 4. This solution is subjected to ion exchange unit for U recovery. The extraction process of U from the carbonate solution via equilibrium batch technique involves two main stages namely adsorption and elution. The uranium adsorption reaction that occurs in the resin can be described by the equation:
Optimization of adsorption stage of uranium
Several experiments were performed using the anion exchange Amerlite IRA400 to determine the optimum conditions of the adsorption process of U from the carbonate solution. These factors include: pH values, stirring time and resin/liquid volume ratios (R/L v/v ratio).
pH value
The effect of different solution pH values upon the loading efficiency using R/L ratio of 0.4/100 (theoretical optimum ratio) and stirring time of 30 min was studied at pH values ranging from 8 up to 10. Results are illustrated in Fig. 5a mean loading efficiency upon the resin reached its maximum value (33.7%) at pH 8.5 which represent the optimum value for U uptake. However, further decrease in pH value has an opposite effect.
Stirring time
To study the effect of stirring time upon the extraction of U from its carbonate solution, a volume of 100 mL leach solution was shaken with 0.4-mL resin (R/L ratio 0.4/100) at pH value 8.5 for different time periods of 20, 30, 40 and 60 min. The obtained data, illustrated in Fig. 5b, indicate that U adsorption efficiency increases from 20 to 43.8% by increasing the stirring time from 20 to 40 min. While further increasing the time up to 60 min, the loading efficiency of U decreased to 41.7% and this may be due to desorption of U. In this context, it is important to mention herein that the lower extraction efficiency of U may be attributed to the uptake of some interfering anions, e.g., SO42−, Cl− and CO32− which compete U upon the resin sites (Morais and Laderia 2008). So, it was decided to increase the volume of resin to improve U extraction efficiency.
Resin/liquid (R/L) ratios
The adsorption efficiency of U was studied at different resin/liquid (R/L) ratios ranging from 0.4/100, 0.8/100, 1/100, 1.2/100 to 1.5/100 at pH 8.5 and stirring time of 40 min. The obtained data Fig. 5c clearly indicate that U adsorption efficiency increased by increasing the resin volumes and achieved the maximum value (99.7%) at R/L ratios of 1.5/100.
Elution process and uranium precipitation
Elution process is not only to regenerate the loaded resin but also to obtain U-rich eluate solution suitable to prepare the preferred U product. After the resin bed was fully saturated with U, it was rapidly washed with suitable volume of distilled H2O to get rid of any impurities and directed to the regeneration process using 100 ml of 10% NaHCO3 solution with stirring for 40 min (Gupta and Singh 2003; Dunn et al. 2008; Robert 2008). The U eluted from the loaded resin attended about 93%. The elution of uranium from the saturated resin bed using sodium bicarbonate is described by the following equation:
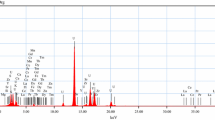
Finally, the obtained U-rich eluate solution of pH 10.2 and assaying (3.05 g/L) was treated with 5% H2SO4 solution to adjust pH to 3.5 and treated with H2O2 solution for U precipitation. About 99% of U was precipitated as UO4∙2H2O at pH 1.5 with stirring time period of 4 h at room temperature (Bhowmik et al. 2009; Kim et al. 2011). After filtration and washing, the precipitated uranyl peroxide cake was ignited at 800 °C for 1 h to be crystallized. The produced U3O8 was identified using EDX analysis technique as shown in Fig. 6, while the purity was already estimated as 97.1% via chemical analysis of its U content.
Finally, the present study effectively achieved the selective extraction for U from carbonaceous shale material and associated REEs and Cu elements using environmentally and more economic chemical reagent (Na2CO3) and extraction via batch technique which can be easily applied in industrial scale
Conclusion
Appropriate selective Na2CO3/NaHCO3 agitation leaching processing has been achieved for the carbonaceous shale of Alloga, southwestern Sinai. Based on the (Ea) values, it can be inferred that the predominant dissolution mechanism of U is diffusion controlled only. Carbonate leach liquor was prepared by applying Na2CO3/NaHCO3 of 10% concentration at 90 °C with stirring time for 2 h, U content assay 0.034g/L. Amberlite IRA400 anion exchange resin in batch experiments was applied for extraction of 99.7% U at pH 8.5 with stirring time 40 min and R/L ratio 1.5/100. U3O8 was finally prepared with purity of 97.1%. The experimental results showed that anion exchange resin method can be one of the important and prospective methods for the recovery of uranium from carbonate solutions relying on environmentally sustainable practices.
References
Abdel Monem AA, El-Assay IE, Hegab OA, El-Fayoumy IF (1997) Gibbsite uranium and copper mineralization, Um Bogma area, Southwestern Sinai, Egypt. J Sedimentol Egypt 5:117–132
Abdellah WM (2014) Extraction of U and valuable elements from Abu Hamata poly-mineralized sedimentary rocks, Southwestern, Sinai, Egypt, Ph.D. Thesis, Chemistry Department, Faculty of Science, Ain Shams University
Abhilash B, Pandey D (2013) Microbially assisted leaching of uranium—a review. Miner Process Extr Metall 34:81–113
Abu Khoziem HA (2017) Mineralogical characteristics and leachability of some valuable metals from Abu Zienema poly mineralized carbonaceous shale, southwestern Sinai, Egypt. J Sedimentol 23:57–70
Al Shami AS (2003) Structural and lithologic controls of U and Cu mineralization of Um Bogma environs, Southwestern Sinai, Egypt, Ph.D. Thesis, Fac. Sc., Mansora Univ. Egypt
Ali HH (2016) Uranium and REEs mineralization of Wadi El Saha area, south western Sinai, Egypt. Ph.D. Thesis, Fac. Sc., Al-Azhar Univ. Egypt
Bhowmik KA, Shanmugavelu P, Dhvamuri D, Agrawal A (2009) Single stage purification of U refining. World Intellected Property Organization: International Bureau, International Publication No. Wo. 2009/013759 A (PCT)
Crundwell FK (2013) The dissolution and leaching of minerals. Mechanisms, myths and misunderstandings. Hydrometallurgy 139:132–148
Dunn G, Vagenas J, Teo YY (2008) Uranium recovery by continuous ion exchange of alkaline leachate. Orway Mineral Consultants (WA) Pty Ltd, Western Australia, Elemental Engineering, Western Australia
El Assy I, Botros NH, Abdel Razik A, El Shamy AS, Ibrahim SK, Sherif HY, Attia KE, Moufei AA (1986) Report on proving of some radioactive occurrences in west central Sinai. Int Rept (N. M. A) Cairo, Egypt
El-Ansary AL, Abd El Wahab GM, Bayomi EE, Nouhb EA (2017) Purification of Abu-Zenima wet crude yellow cake using alkaline leaching of U(VI). Egypt J Pet (in press). https://www.sciencedirect.com/science/article/pii/S1110062117301927
El-Sheikh EM, Ali S, Ghazala R, Abdel Warith A, Salem F (2015) Leaching characteristic of uranium and copper from their mineralization in the carbonate rich latosol of Abo Thor locality, SW Sinai, Egypt. J Isot Radiat Res 47(2):231–246
Gupta K, Singh H (2003) Uranium resource processing, Secondary resources. Springer, Trombay
Hunter E. (2013) On the leaching behavior of uranium bearing resources in carbonate–bicarbonate solution by gaseous oxidants. Ph.D. thesis. Faculty and the Board of Trustees of the Colorado. School of Mines (Mining and Earth Systems Engineering), 283
Jaireth S, McKay A, Lambert L (2008) Association of large sandstone uranium deposits with hydrocarbons, AUSGEO, issue 89 March
Kim KW, Hyun JT, Lee KY, Lee EH, Lee KW, Song KC, Moon JK (2011) Effects of the different conditions of uranyl and hydrogen peroxide solutions on the behaviour of the uranium peroxide precipitation. J Hazard Mater 193:8–52
Kuşcu M, Özsoy R, Özçelik O, Altunsoy M (2016) Trace and rare earth element geochemistry of black shales in Triassic Kasımlar Formation, Anamas-Akseki Platform, Western Taurids, Turkey. In: World Multidisciplinary Earth Sciences Symposium (WMESS). IOP conference series: earth and environmental science, 44
Ladeira ACQ, Morais CA (2005) Effect of ammonium, carbonate and fluoride concentration on the uranium recovery by resins. Radiochim Acta 93:207–209
Levenspiel O (1999) Chemical reaction engineering. Wiley, New York
Li H, Zhao C, Yong W, Qiang D (2006) Industrial test of alkaline heap leaching at uranium deposit of Lantian Uranium Mine. Uranium mining and metallurgy, vol 25, no 01. Lantian Uranium Mine, CNNC, Xi’an, Peoples Republic of China, Youkuangye, 25(1):9–14
Lunt D, Boshoff P, Boylett M, El-Ansary Z (2007) Uranium extraction: the key process drivers. J South Afr Inst Min Metall 107:420–426
Marczenko Z (2000) Spectrophotometric determination of elements. Wiley, Harwood
Mathew KJ, Burger S, Ogt SV, Mason PM, Narayanan UI (2009) Uranium assay determination using Davies and Gray titration. In: Processing the eighth international conference on methods and applications of radioanalytical Chemistry (Marc Viii) Kaailua-Kona, Hawaii, 5
Morais CA, Laderia AC (2008) The influence of competitive species on uranium recovery using resin and solvent extraction techniques. In: Young PR, Anderson CG, Choi Y (eds) Hydrometallurgy. SME Phoenix, Arizona, pp 292–296
Muhammad A, Younas M, Rezakazemi M (2017) Quasi-dynamic modeling of dispersion-free extraction of aroma compounds using hollow fiber membrane contactor. Chem Eng Res Des 127:52–61
Nascimento MRL, Fatibello-Filho O, Teixeira LA (2004) Recovery of uranium from acid mine drainage waters by ion exchange. Miner Process Extr Metall Rev 25:129–142
Oraby A, El- Skeikh E, Salah W, El- Saied F, El Gendy H, Ismaiel D (2018) Recovery of uranium and copper from mineralized Dolostone, Gabal Alluga, southwestern Sinai, Egypt. J Radiat Res Appl Sci (under press)
Robert SS (2008) Hydrometallurgy processing of the sixth international symposium, society of mining. Metallurgy and Exploration, Inc, USA
Santos EA, Ladeira AC (2011) Recovery of uranium from mine waste by leaching with carbonate-based reagents. Environ Sci Technol 45(8):3591–3597
Seneda JA, Figueiredo FF, Abrao A, Carvalho FMS, Frajndlich UC (2001) Recovery of uranium from the filtrate of ammonium diuranate prepared from uranium hexafluoride. J Alloys Compd 323–324:838–841
Shapiro L, Barnnock WW (1962) Rapid analysis of silicate, carbonate and phosphate rocks. US Geol Surv, Bull, p 1114
Suri AK, Ghosh SK, Padmanabhan NPH (2009) International symposium on uranium raw material for the nuclear fuel cycle, Vienna, pp 231–246
Acknowledgements
The authors would like to express their appreciation to Prof. T. E. Amer, (NMA), and Prof. Abd El Wahab, G. M (NMA) for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Editorial handling: Inamuddin.
Rights and permissions
About this article
Cite this article
Abdel Wahab, S., Rezik, A., Abu Khoziem, H.A. et al. Kinetics of uranium carbonate leaching process from carbonaceous shale, southwestern Sinai, Egypt. Euro-Mediterr J Environ Integr 4, 19 (2019). https://doi.org/10.1007/s41207-019-0106-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41207-019-0106-0