Abstract
A total of 249 cow and 33 goat milk samples were collected in rural areas of Croatia during the period 2010–2014. Lead concentrations in milk samples were analyzed by graphite furnace-atomic absorption spectroscopy. Mean Pb concentrations in milk ranged from (μg/kg): cow 10.8–12.2; goat 9.33–60.0. The highest Pb level of 131 μg/kg in cow milk was measured during 2014. There were no significant differences in Pb levels between cow and goat milk and also in goat milk among the analysed years. However, significant differences were found in cow milk among years. The highest Pb was determined in 2011 (157 μg/kg in goat milk). The calculated estimated weekly intakes of Pb concentrations for cow and goat milk contribute only 1.37 % and 1.84 % to the provisional tolerable weekly intake. Therefore, the consumption of milk from both species should not pose a consumer health risk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
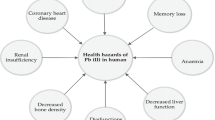
Milk is one of the basic components required in the human diet, especially with regard to its protein and mineral composition. Therefore, it is necessary to monitor toxic substances and metals in milk that can enter the food chain as a result of environmental contamination. It is known that milk and dairy products may contain different concentrations of toxic metals, due to the geological background and environment releases from various industrial processes (Ataro et al. 2008). Toxic metal contamination in milk has been reported in polluted industrial areas in highly industrialized countries such as Turkey, India, Pakistan or Brazil (Simsek et al. 2000; Patra et al. 2008; Javed et al. 2009; Soares et al. 2010). It is known that a chronic lower level accumulation of toxic metals, such as Cd and Pb, has adverse affects on human and animal health (Satarug et al. 2003). Continuous absorption of small amounts of Pb in humans may cause serious health issues in growing children, including mental development disorders such as reading and learning disabilities and deficiencies in concentration, hearing degradation, adverse effects on kidney function, hematopoietic system through restraining the synthesis of hemoglobin and causes anemia, and the cardiovascular system (Rahimi 2013). Accordingly, controlling Pb levels in milk in accordance with the established maximum residue levels (MRL) of 20 μg/kg set by the EU is imperative (EC 2006).
Today in Croatia, industrialization is not on the rise; instead, a decline in industrial development has been recorded. Few studies have been conducted on the toxic metal concentrations in raw cow and goat milk in Croatia (Sikirić et al. 2003; Bilandžić et al. 2011). Goat milk is still not widely produced in the European Union and in Croatia. The total world production of goat milk accounts for about 2.4 % of all milk, of which 58 % is produced in Asia, 24 % in Africa and 15 % in Europe (Dermit et al. 2014). However, there is great interest for the production of goat milk due to the composition and high quality of the product, particularly for population groups with particular needs, such as infants and children suffering from hypersensitivity to cow milk, and the elderly (Kondyli et al. 2007). Previous research showed a higher content of macroelements and vitamins, and also better digestibility, alkalinity, buffering capacity and certain therapeutic benefits in goat milk than in cow milk (Cashman 2003; Navarro-Alarcón et al. 2011).
The aim of the present study was to assess differences in Pb concentrations in raw cow and goat milk in the period from 2010 to 2014, and to compare the results with the literature data. Furthermore, the estimated weekly intake for Pb was calculated and compared with the toxicological value for the purpose of risk assessment.
Materials and Methods
During the period 2010–2014, a total of 249 cow and 33 goat milk samples (500 mL) were collected at small dairy farms from rural areas in seven counties in continental Croatia (Fig. 1): Sisak-Moslavina (SM), Brod-Posavina (BP), Vukovar-Srijem (VS), Bjelovar-Bilogora (BB), Požega-Slavonia (PS), Virovitica-Podravina (VP) and Osijek-Baranja (OB). Milk samples were collected as part of the National Residue Monitoring Plan on small farms (mainly with 3–15 cows and/or goats) where cows and goat graze freely in the surrounding pastures or use hay in the traditional way. During the study period, milk samples were collected from individual cows and goats between May and October. Upon collection, all samples were placed into clean, acid-washed polyethylene bottles, labelled and stored at −18 °C until analysis.
All reagents, HNO3, H2O2, and HCl, were of analytical reagent grade (Analytical Grade, Kemika, Croatia). Ultra pure water (18.2 MΩ/cm resistivity, Direct-Q® 5 UV, Millipore, USA) was used for all dilutions. Plastic and glassware were cleaned by soaking in diluted HNO3 (1/9, v/v) and rinsed with ultra pure water prior to use.
Standard stock solution for Pb of 1000 mg/L was supplied by Perkin Elmer (Waltham, MA, USA). The matrix modifier used in each atomization for Pb was 0.003 mg Mg(NO3)2 (Perkin Elmer, Waltham, MA, USA). The calibration curve was prepared at different concentrations and contained four calibration points.
Samples (2 g) were weighed in a PFA digestion vessel and 1 mL H2O2 (30 %, v/v) and 6 mL HNO3 (65 %, v/v) were added. A high-pressure laboratory microwave oven (Multiwave 3000, Anton Paar, Ostfildern, Germany) was employed to perform the acid digestion of samples. The digestion program was: step 1 power 800 W, ramped 15 min, 800 W for 15 min; step 2 power 0 W for 15 min. Each digested sample was diluted to a final volume of 25 mL with ultra-pure water.
Detection limits were determined as the concentration corresponding to three times the standard deviation calculated for ten blank milk samples. All specimens were run in batches that included blanks, a standard calibration curve, two spiked specimens, and one duplicate. The quality of data was checked by the analysis of the recovery rate using the certified reference material, skim milk powder (BCR-151, IRMM, Geel, Belgium).
The analysis of Pb was conducted using graphite furnace-atomic absorption spectroscopy by AAnalyst 600 (Perkin Elmer, Waltham, MA, USA). Argon was used as the inert gas. Pyrolytically coated graphite tubes with a platform were used. Measurements (integrated absorbance peak areas) were carried out using single element hollow lamps. The instrument settings and optimising temperature programs of the spectrometer are summarised in Table 1.
The estimated daily intake (EDI) of Pb was calculated by the equation:
where C is the metal concentration (µg/kg w.w.), MS is the meal size (kg), and BW is body weight (kg). All consumption limits and risk factors were calculated for adults assuming a body weight (BW) of 70 kg.
The estimated weekly intake (EWIs, μg/week b.w.) was calculated by the equation:
The average consumption of milk in Croatia was set as 0.3 L/day per adult (Antonić Degač et al. 2007). The value of EWI was used to calculate contributions of Pb to the toxicological value (PTWI, provisional tolerable weekly intake).
Statistical analyzes were performed using STATA® 13.1 (StataCorp LP®, College Station, TX, USA). The concentrations of Pb in milk samples were expressed as the minimum and maximum, mean ± standard deviation (SD).
The Shapiro–Wilk W test was applied to determine the distribution of the data. The test results showed that data were not normally distributed. The Wilcoxon rank-sum test was used to test for statistical differences in Pb levels between: all data (all years) for cow milk and all data for goat milk, studied years for both types of milk, two types of milk in same year of observation. Regarding the small number of goat milk samples during 2010 and 2011, the results of these 2 years were used only in the comparison of all data (all years) with all data of cow milk, but not in tests between examined years. Statistically significant differences were expressed at the level of probability of 0.05 and 0.01.
Results and Discussion
The accuracy of Pb analysis was tested by analyzing the standard reference material. The recovery rate was 97.5 % (n = 5). The limit of detection (LOD) in milk was 4.0 μg/kg. The Pb concentrations in cow and goat milk samples from Croatia are reported in Table 2.
During the period 2010–2014 Pb concentrations were measured in the range 10.8–12.2 μg/kg in cow milk and 9.33–60.0 μg/kg in goat milk. The highest Pb level of 131 μg/kg in cow milk was measured during 2014.
The pooling of all Pb results into two groups showed no significant difference between cow and goat milk. Also, there were no significant differences between cow and goat milk during 2012, 2013 and 2014. Comparison of the Pb concentrations in cow milk between the studied years showed significant differences between: 2010 and 2012 (p < 0.01), 2011 and 2013 (p < 0.05), 2011 and 2014 (p < 0.05), 2012 and 2013 (p < 0.01), 2012 and 2014 (p < 0.01). However, there were no significant differences of the Pb concentrations for goat milk between the studied years.
Mean Pb concentration determined in 2011 in goat milk was 60.0 μg/kg, while the maximum concentration was 157 μg/kg (Table 2). Regarding goat milk, there are few literature data for toxic metal concentrations. In comparison to the present results, lower Pb levels of 7.37 μg/L were recently reported in Iran (Rahimi 2013). However, very high Pb concentrations of 42.69 mg/L were found in industrial areas in Pakistan (Javed et al. 2009). The influence of Pb contamination in the environment is demonstrated by the high Pb levels in the blood of goats in Spain grazing near highways or mining areas, which were 2.2–4.5 higher than in animals grazing in rural regions (García-Fernández et al. 2003).
In previous studies regarding Pb concentrations in cow milk in Croatia, higher Pb concentrations than those in this study were measured in the range of 23.06–70.56 μg/kg (Sikirić et al. 2003). Also, mean Pb concentrations determined in the northern and southern regions of Croatia were much higher, 58.7 and 36.2 μg/L (Bilandžić et al. 2011). In 35.5 % samples from northern and 28.3 % samples from southern regions, levels exceeded the MRL value of 20 μg/kg. However, in the present study, only one sample each of cow and goat milk had concentrations exceeding the MRL value. These results are due to the significant decline in industrialization in Croatia. It can be assumed that low Pb concentrations in milk are certainly due to the fact that grazing pastures are located in rural areas and away from the main road routes.
The concentrations found in the present study were similar to levels found in cow milk from Italy in the range of 0.1–9.92 μg/kg (Licata et al. 2004), from Iran in the range of 7.9–9.88 μg/L (Tajkarimi et al. 2008; Rahimi 2013), or to levels from 8.00 to 19.7 μg/kg in South Africa (Ataro et al. 2008), and up to 14.4 μg/kg in Brazil (Nardi et al. 2009). However, lower Pb levels in cow milk were measured in Romania (0.04–0.16 μg/kg; Birghila et al. 2008), France (6 μg/kg; Arnich et al. 2012) and Spain (5.23 and 0.516 μg/L; Sola-Larrańaga and Navarro-Blasco 2009; Rey-Crespo et al. 2013).
It has been suggested that Pb may be present in milk due to accumulation along roads and motorways, various industrial activities, contaminated water irrigation systems, use of pesticides and climatic factors (Licata et al. 2004; Tajkarimi et al. 2008). Very high Pb concentrations were reported in cow milk collected from polluted industrial and busy traffic areas in Turkey (0.049 and 0.032 mg/kg; Simsek et al. 2000), or industrial plants and smelters in India (0.65–0.85 mg/L; Patra et al. 2008), Pakistan (21.78 and 15.96 mg/L; Javed et al. 2009) and Brazil (0.23 mg/L; Soares et al. 2010). In a recent study in Serbia, high Pb levels were found in the range 54.3–91 μg/kg (Suturović et al. 2014).
In the present study, the estimated daily and weekly intake (EDI, EWI) was calculated and compared with the toxicological value for the purpose of a risk assessment of Pb concentrations in milk. World Health Organization (WHO) and Food and Agriculture Organization (FAO) have established a provisional tolerable weekly intake (PTWI) level for the purpose of estimating the potential risk to human health derived from the ingestion and accumulation of major toxic elements on a weekly basis. The PTWI for Pb is set at 25 μg/kg b.w. (FAO/WHO 1993). Table 3 presents the calculated EWI values for the total mean Pb values for cow and goat milk and their contribution to the PTWI value. Contributions of Pb concentrations for cow and goat milk were similar and were <2 %. Therefore, the consumption of milk from both species does not seem to pose a risk to the normal consumer with regard to Pb levels.
In conclusion, this study supports the findings that the absence or reduction of industrial plants and the use of pastures distant from main motorways and roads greatly reduces environmental contamination with Pb, which is reflected in low concentrations found in food or milk.
References
Antonić Degač K, Laido Z, Kaić-Rak A (2007) Obilježja prehrane i uhranjenosti stanovništva Hrvatske. Hrvatski zavod za javno zdravstvo. Hrvatski časopis za javno zdravstvo 3:9
Arnich N, Sirot V, Rivière G, Jean J, Noël L, Guérin T, Leblanc J-L (2012) Dietary exposure to trace elements and health risk assessment in the 2nd French total diet study. Food Chem Toxicol 50:2432–2449
Ataro A, McCrindle RI, Botha BM, McCrindle CME, Ndibewu PP (2008) Quantification of trace elements in raw cow’s milk by inductively coupled plasma mass spectrometry (ICP-MS). Food Chem 111:243–248
Bilandžić N, Đokić M, Sedak M, Solomun Kolanović B, Varenina I, Knežević Z, Benić M (2011) Trace element levels in raw milk from northern and southern regions of Croatia. Food Chem 127:63–66
Birghila S, Dobrinas S, Stanciu G, Soceanu A (2008) determination of major and minor elements in milk through ICP-AES. Environ Eng Manag J 7:805–808
Cashman KD (2003) Minerals in dairy products. In: Roginski H, Fuquay JW, Fox PF (eds) Encyclopaedia of dairy sciences. Academic Press, London, pp 2051–2065
Dermit FZ, Mikulec N, Bendelja LJD, Antunac N (2014) Nutritional and therapeutic properties of goat’s milk. Mljekarstvo 64:280–286
EC (2006) European Commission, Regulation (EC) no. 1881 (2006): 19 of December 2006, setting maximum levels for certain contaminants in foodstuffs. Off J L364:5–24
FAO/WHO (1993) Evaluation of certain food additives and contaminants; 41st report of the joint FAO/WHO expert committee on food additives. Technical Report Series, No. 837, World Health Organization, Geneva, Switzerland
García-Fernández AJ, Navas I, Romero D, Gomez-Zapata M, Luna A (2003) Influence of leaded-gasoline regulations on the blood lead concentrations in Murciano-Granadina goats from Murcia Region, Southeast Spain. Bull Environ Contam Toxicol 70:1178–1183
Javed I, Jan I, Muhammad F, Rahman Z, Khan MZ, Aslam B, Sultan JI (2009) Heavy metal residues in the milk of cattle and goats during winter season. Bull Environ Contam Toxicol 82:616–620
Kondyli E, Katsiari MC, Voutsinas LP (2007) Variations of vitamins and mineral content in raw goat milk of the indigenous Greek breed during lactation. Food Chem 100:226–230
Licata P, Trombetta D, Cristani M, Giofre F, Martino D, Calo M, Naccari F (2004) Levels of “toxic” and “essential” metals in samples of bovine milk from various dairy farms in Calabria, Italy. Environ Int 30:1–6
Nardi EP, Evangelista FS, Tormen L, Saint´Pierre TD, Curtius AJ, de Souza SS, Barbosa F (2009) The use of inductively coupled plasma mass spectrometry (ICP-MS) for the determination of toxic and essential elements in different types of food samples. Food Chem 112:727–732
Navarro-Alarcón M, Cabrera-Vique C, Ruiz-López MD, Olalla M, Artacho R, Giménez R, Quintana V, Bergillos T (2011) Levels of Se, Zn, Mg and Ca in commercial goat and cow milk fermented products: relationship with their chemical composition and probiotic starter culture. Food Chem 129:1126–1131
Patra RC, Swarup D, Kumar P, Nandi D, Naresh R, Ali SL (2008) Milk trace elements in lactating cows environmentally exposed to higher level of lead and cadmium around different industrial units. Sci Total Environ 404:36–43
Rahimi E (2013) Lead and cadmium concentrations in goat, cow, sheep, and buffalo milks from different regions of Iran. Food Chem 136:389–391
Rey-Crespo K, Miranda M, López-Alonso M (2013) Essential trace and toxic element concentrations in organic and conventional milk in NW Spain. Food Chem Toxicol 55:513–518
Satarug S, Baker JR, Urbenjapol S, Haswell-Elkins M, Reilly PE, Williams DJ (2003) Global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicol Lett 137:65–83
Sikirić M, Brajenović N, Pavlović I, Havranek JL, Plavljanić N (2003) Determination of metals in cow’s milk by flame atomic absorption spectrophotometry. Czech J Anim Sci 48:481–486
Simsek O, Gultekin R, Oksuz O, Kurultay S (2000) The effect of environmental pollution on the heavy metal content of raw milk. Nahr Food 44:360–371
Soares VA, Kus MMM, Peixoto ALC, Carrocci JS, Salazar RFS, Filho HJI (2010) Determination of nutritional and toxic elements in pasteurized bovine milk from Vale do Paraiba region (Brazil). Food Control 21:45–49
Sola-Larrańaga C, Navarro-Blasco I (2009) Chemometric analysis of minerals and trace elements in raw cow milk from the community of Navarra, Spain. Food Chem 112:189–196
Suturović Z, Kravić S, Milanović S, Ðurović A, Brezo T (2014) Determination of heavy metals in milk and fermented milk products by potentiometric stripping analysis with constant inverse current in the analytical step. Food Chem 155:120–125
Tajkarimi M, Faghih MA, Poursoltani H, Nejad AS, Motallebi AA, Mahdavi H (2008) Lead residue levels in raw milk from different regions of Iran. Food Control 19:495–498
Acknowledgments
The authors are thankful to Marijana Fluka and Mirjana Hren for assistance in sample preparation. This study was supported with a grant from the Croatian Veterinary Institute, Zagreb, Croatia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bilandžić, N., Sedak, M., Čalopek, B. et al. Lead Concentrations in Raw Cow and Goat Milk Collected in Rural Areas of Croatia from 2010 to 2014. Bull Environ Contam Toxicol 96, 645–649 (2016). https://doi.org/10.1007/s00128-016-1749-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-016-1749-z