Abstract
Nuclear grade zirconium and hafnium are important materials in nuclear power plants, which are usually produced with solvent extraction and separation technology. In this paper, the preferential extracting hafnium was successfully achieved by the selective complexation of the selected organic acids. Hydrochloric acid concentration, organic acid concentration, D2EHPA concentration were investigated to explore the optimum extraction conditions. Increasing acidity and extractant concentration was not conducive to the separation of zirconium and hafnium, while organic acid could effectively improve the separation factor, which was verified by ATR-FTIR spectroscopy. The largest separation factor, 9.936, for hafnium over zirconium was obtained.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Zirconium and hafnium co-exist in nature in the form of ores [1]. Namely, there are no separate zirconium and hafnium ores. The content of zirconium in the earth’s crust is 0.025%, and hafnium is approximately 2% of zirconium [2, 3]. They have nearly identical electronegativity, atomic radius and ionic radius [4]. On account of the similarity, the separation of zirconium and hafnium is difficult to achieve. Zirconium and hafnium play crucial roles and have completely different applications in the nuclear power industry [5,6,7]. Zirconium is mainly used as fuel cladding material, while hafnium is used to control the rate of reaction. The nuclear grade zirconium and hafnium are strictly required for purity [8, 9]. As a result, studying the separation of zirconium and hafnium in depth is imperative [10].
The solvent extraction method in wet separation is widely used in the separation of zirconium and hafnium because of its low cost, high yield and thorough separation [1, 2, 11, 12]. Solvating extractants such as tri-n-butyl phosphate (TBP), tri-n-octylphosphine oxide (Cyanex 921), mixture of straight chain alkylated phosphine oxides (Cyanex 923), mixture of branched chain alkylated phosphine oxides (Cyanex 925) and tri-n-octyl phosphine oxide (TOPO) are frequently found in the extraction studies of zirconium and hafnium [13]. Banda et al. [14] employed TOPO as solvating extractant to discern the effect of acidity and synergetic agents on the extraction of zirconium and hafnium in acidic chloride solution. 2.5–3 mol dm−3 HCl condition was confirmed to be effective in extracting zirconium from hafnium. Di(2-ethylhexyl)phosphoric acid (D2EHPA), 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester (PC 88A), 5,8-diethyl-7-hydroxy-6-dodecanone oxime (LIX 63), bis(2,4,4-trimethylpentyl)phosphinic acid (Cyanex 272), bis(2,4,4-trimethylpentyl)dithiophosphinic acid (Cyanex 301), bis(2,4,4-trimethylpentyl)monothiophosphinic acid (Cyanex 302) and alkyl monocarboxylic acid (Versatic Acid 10) are especially widely applied to extract zirconium and hafnium as acidic extractants [15, 16]. Lee et al. [17] explored the stoichiometry of zirconium and hafnium in hydrochloric acid system, using Versatic Acid 10 as extractant. The results clearly demonstrated that zirconium was extracted in the form of ZrO2+ and combined with two molecules of extractant. In contrast, Hf4+ rather than HfO2+ was extracted into organic phase. Furthermore, the separation of zirconium and hafnium can also be achieved by alkaline extractants, such as tri-iso-octylamine (Alamine 308), mixture of tertiary aliphatic amines (Alamine 336), methyl trioctyl ammonium chloride (Aliquat 336), tri(2-ethylhexyl)amine (TEHA) and tri-n-octylamine (TOA). Banda et al. [18] investigated several amine-based extractants in hydrochloric acid medium and Alamine 336 presented the highest separation factor of zirconium over hafnium. After scrubbing hafnium with dilute H2SO4 solution, zirconium was entirely stripped into aqueous phase by repeatedly contacting with 1 mol dm−3 HCl. All of the above systems have a strong extraction capacity for zirconium and require a large number of complicated operations to obtain hafnium with nuclear purity.
Considering the small amount of hafnium in zirconium ore, exploring the method which preferentially extracts hafnium is of practical importance. Due to the nature of the zirconium-hafnium ion, the above extraction systems preferentially extract zirconium and the separation factors are not large [3, 6,7,8, 14, 18,19,20]. In a few reports on the preferential extraction of hafnium, the separation of zirconium and hafnium is not ideal and the separation factors are low. Xu et al. [21] reported a process to separate the two metals by the mixture of di-isobutyl ketone (DIBK) and TBP in HSCN, in which hafnium was enriched in the organic phase and zirconium remained in the aqueous phase. The methyl isobutyl ketone (MIBK)-NH4SCN system which is used in large-scale industrial production also gives priority to the extraction of hafnium, because SCN− has better affinity for hafnium than zirconium [4, 22]. However, HSCN complex is harmful to the environment [23]. Therefore, it is an inevitable trend to explore novel extraction systems with the preferential extraction of hafnium and environmental protection.
Acidic organophosphorus extractants have good stability and are widely used in hydrometallurgy. Nowadays, D2EHPA, PC 88A and Cyanex 272 especially become the main research hotspots [1, 2, 4, 6, 7, 19, 24,25,26]. The most striking difference between them is the number of alkoxy groups on the phosphorus atom, leading to the change in pKa value of extractant and activity of the functional group P(O)OH [7, 27]. Banda et al. [1] reported that PC 88A and D2EHPA selectively extracted hafnium over zirconium in sulfuric acid system and the separation factor was not low. This phenomenon is due to the stronger complexation ability of sulfate anion with zirconium than hafnium. Reddy et al. [25] found that PC 88A had the opportunity to preferentially extract hafnium over zirconium when the acidity was low. The addition of tartaric acid to aqueous phase inhibited the extraction of zirconium by D2EHPA, nevertheless, the extraction rate of hafnium remained unchanged [28].
Zirconium oxychloride products containing hafnium are produced in large quantities especially in China. As a result, it is of great significance to study the preferential separation of hafnium in the chloride system. In this paper, we selected citric acid, tartaric acid and glutamic acid as zirconium complexing agents and D2EHPA as extractant to explore their influence on the separation of zirconium and hafnium in hydrochloric acid. The optimum conditions for preferential extraction of hafnium were studied. Under the optimized conditions, the highest separation coefficient so far was obtained.
Materials and methods
Chemicals and reagents
D2EHPA (Purity > 98%) was purchased without further purification. All other chemicals (Sinopharm, China) were of analytical grade. 90% octane-10% octanol was used as diluent in which octanol could effectively eliminate the formation of the third phase.
The mixed solution of zirconium and hafnium was prepared by dissolving ZrOCl2∙8H2O and HfCl4 in corresponding acid solution. Hydrochloric acid was used to adjust acidity in the range of 0.06–0.14 mol dm−3. To ensure the accuracy of extraction experiment, the feed solution of zirconium and hafnium was freshly prepared to avoid hydrolysis and polymerization [4].
Experimental procedures
2 ml of aqueous and organic phases were shaken for 1 h at ambient temperature in a water-bath oscillator until they were in equilibrium. Rotate speed was maintained at 300 rpm. After centrifugation and separation, the concentrations of zirconium and hafnium in the aqueous phase were measured by Inductively Coupled Plasma Mass Spectrometry (ICP-MS, Thermo Scientific, X Series, America). The concentrations of metals in the organic phase were calculated by mass balance. The value of distribution coefficient (D) was taken as the ratio of the concentration of metal in the organic phase to that in the aqueous phase at equilibrium [1]. The extraction percentage (E) and separation factor (SF or β) were obtained by the formulas E (%) = D × 100/(D + 1) and β = DHf/DZr.
Fourier transform infrared (FTIR, Bruker Vertex 700, America) with an attenuated total reflection (ATR, Bruker, America) accessory was used to measure the infrared spectra of liquid complexes. With water as the background, wavelengths ranging from 1200 to 2000 cm−1 were recorded.
Results and discussion
The influence of HCl concentration on the separation of zirconium and hafnium
There are two main dynamic equilibrium processes: complex equilibrium between organic acids and metal ions, and extraction equilibrium between D2EHPA and metal ions. As an acidic extractant, D2EHPA follows the cation exchange mechanism in low acidity [25]. In the absence of organic acid, the distribution coefficient should decrease with increasing acidity. On the other hand, the dissociation degree of the organic acid decreases with the increasing acidity and the concentration of organic acid anions involved in complexation with zirconium and hafnium, which promotes the extraction by D2EHPA to some extent. Therefore, complex equilibrium and extraction equilibrium play the opposite role, and the two factors are competitive.
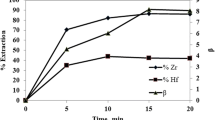
The effect of hydrochloric acid concentration on the extraction of zirconium and hafnium by D2EHPA in the presence of organic acid was investigated in the range from 0.06 to 0.14 mol dm−3. According to the Fig. 1, the distribution coefficient of zirconium and hafnium by D2EHPA increased with the increase of aqueous hydrochloric acid concentration in the tartaric acid or citric acid system. As a consequence, in the competition of extraction and complexation, the complexation ability is stronger than the extraction ability. The separation factor reached the maximum values of 3.45 for tartaric acid and 3.31 for citric acid at the lowest acidity tested, respectively. The test under the same conditions in the absence of organic acid indicated that the separation factor was only 2.02. The results show that the organic acids employed have a stronger complexation effect on hafnium than zirconium. Zirconium easily combines more organic acids to form complex anion than hafnium. However, in the organic phase, D2EHPA as an acidic extractant loses hydrogen ion and exhibits a negative valence, which cannot be combined with complex anion, so that hafnium is more easily extracted into the organic phase than zirconium.
In the presence of glutamic acid, the trend of the distribution ratio of zirconium and hafnium showed an opposite characteristic from the above two organic acid systems. The extraction distribution ratio decreased as the acidity increased. When glutamic acid and metal ions coordinate, amino group combines with hydrogen ion to form an ammonia cation (R-NH3+). At the water–oil interface, this structure easily combines with D2EHPA which loses hydrogen ion to form a relatively stable structure. More complexes of metals with glutamic acid are extracted into the organic phase. So in the presence of glutamic acid, the complexing ability is less than the extraction ability. As can be seen from Fig. 1, the distribution coefficient of zirconium and hafnium was much greater when glutamic acid was added to the aqueous phase than the other two organic acids were added, which was consistent with the above ratiocination. The trend of the separation factor was similar with tartaric acid and citric acid, of a maximum about 3.84.
The increased separation factor between Zr and Hf is attributed to their different ability to coordinate with organic acids. The electrons on the 4f orbit of hafnium have an inhibition effect on the bonding. The 4f orbit of the zirconium ion is empty, which facilitates bonding to form a stable complex [29]. As a result, the organic acids can form stable complexes with zirconium ion, whereas the complexes formed by the hafnium ion with organic acids are less stable than zirconium ion which makes the organic acids have a certain promoting effect on the extraction of hafnium over zirconium.
The influence of organic acid concentration on the separation of zirconium and hafnium
Zirconium and hafnium ions have empty orbitals and the stability constants of some inorganic zirconium and hafnium complexes are increasing in the order: OH− > F− > SO4− > NO3− > Cl− > ClO4− [2, 4]. The ability of citric acid and trioxyglutaric acid to complex with zirconium and hafnium is higher than that of sulfate ion and far greater than the chloride ion [29]. This means that organic acids are strongly capable of complexing with zirconium and hafnium.
Based on Fig. 1, we chose the acidity (0.06 mol dm−3) corresponding to the largest separation factor to investigate the effect of organic acid concentration on the separation of zirconium and hafnium. According to Fig. 2, with the addition of organic acids, the extraction percentage of zirconium and hafnium gradually decreased, which was attributed to the complexation of organic compounds in the aqueous phase. It is noteworthy that the separation factor of hafnium over zirconium increased gradually with the same acidity.
In other words, organic acids inhibit the extraction, and have stronger complex ability with zirconium than hafnium, which leads to the increasing separation factor of hafnium over zirconium. The maximum separation factor of 5.51 was obtained in 0.007 mol dm−3 tartaric acid system.
The influence of extractant concentration on the separation of zirconium and hafnium
The influence of D2EHPA concentration within 0–0.02 mol dm−3 on the extraction of zirconium and hafnium has been explored from the aqueous solutions containing HCl of 0.06 mol dm−3 and organic acid of 0.007 mol dm−3. The experimental results are shown in Fig. 3. The change of D2EHPA concentration has a great effect on the separation of zirconium and hafnium. The extraction capacity was improved effectively with the increase of the concentration of D2EHPA. The separation factor decreased with more zirconium extracted into the organic phase. High concentration of extractant is not beneficial to the preferential extraction of hafnium. In the case of 0.002 mol dm−3 D2EHPA, 0.06 mol dm−3 HCl, 0.07 mol dm−3 organic acid, separation factor β reached 7.892, 7.463, 9.932 for tartaric acid, citric acid and glutamic acid, respectively. This is a marked improvement over the previously reported data.
Through the above results, it is found that organic acid has a positive influence on preferential extraction of hafnium over zirconium, and the separation factor can be obviously increased in the presence of organic acids with enough large complexing ability.
The ATR-FTIR spectroscopy of zirconium and hafnium complexes with tartaric acid
Because the species of zirconium and hafnium ions are so complex in aqueous solution that there are few reported methods for determining their complexing constants with organic acids [30].
To prove the above deduction more clearly, we have done a set of ATR-FTIR measurements, choosing tartaric acid as organic acid.
In different samples, the concentrations of zirconium/hafnium and tartaric acid maintained at 0.01 and 0.03 mol dm−3, respectively. Hydrochloric acid of 0.06 mol dm−3 was selected as solvent to inhibit hydrolysis. A strong absorption peak was observed at 1650 cm−1 which was attributed to stretching vibration of free COO− in the solution. As can be seen from the Fig. 4, the strength of the peak at 1650 cm−1 was hafnium-tartaric acid > zirconium-tartaric acid > tartaric acid. Tartaric acid is a weak acid that can only ionize a small amount of H+ and COO− without interference from other media. The addition of zirconium and hafnium ions promotes the dissociation of tartaric acid. Zirconium was easy to coordinate with tartaric acid so that the free COO− was reduced. By contrast, hafnium had a weaker coordination capacity with tartaric acid than zirconium, so the peak intensity of COO− was highest.
In consequence, D2EHPA can achieve the effect of preferential extraction of hafnium by means of organic acids. The above conclusions are consistent with the results of the extraction experiment.
Conclusions
After the preliminary study on extraction and separation of zirconium and hafnium with organic acids by D2EHPA in the system of hydrochloric acid, we found that the organic acids could reduce the distribution coefficient of zirconium and hafnium. At the same time, the complexation ability of zirconium was stronger than that of hafnium, so the separation factor of hafnium over zirconium was improved. Reducing the acidity and the concentration of extractant, increasing the amount of organic acids helped to selectively extract hafnium from the mixture of zirconium and hafnium. When the concentration of HCl, glumatic acid and D2EHPA was 0.06, 0.007, 0.002 mol dm−3, the largest separation factor, 9.936, in this experiment appeared. The results of ATR-FTIR spectroscopy indicated that the binding capacity of tartaric acid and zirconium was stronger, thus promoting the extraction of hafnium with D2EHPA. Organic acids show actual application prospect on the separation of zirconium and hafnium.
References
Banda R, Min SH, Lee MS (2013) Selective extraction of Hf(IV) over Zr(IV) from aqueous H2SO4 solutions by solvent extraction with acidic organophosphorous based extractants. J Chem Technol Biotechnol 89(11):1712–1719
Wang LY, Lee MS (2016) Solvent extraction reaction of hafnium (IV) from strong sulfuric acid solutions with D2EHPA and PC 88A. Sep Sci Technol 51(5):759–766
Banda R, Lee HY, Lee MS (2013) Separation of Zr and Hf from strong hydrochloric acid solution by solvent extraction with TEHA. J Radioanal Nucl Chem 295(2):1537–1543
Wang LY, Lee MS (2016) A review on the aqueous chemistry of Zr (IV) and Hf(IV) and their separation by solvent extraction. J Ind Eng Chem 39:1–9
Yang XJ, Fane AG, Pin C (2002) Separation of zirconium and hafnium using hollow fibers: part I. Supported liquid membranes. Chem Eng J 88(1–3):37–44
Xu ZG, Wang LJ, Wu M, Xu YL, Chi R, Li PH, Zhao J (2016) Separation of zirconium and hafnium by solvent extraction using mixture of DIBK and P204. Hydrometallurgy 165:275–281
Wang LY, Lee HY, Lee MS (2015) Solvent extraction separation of Zr and Hf from nitric acid solutions by PC88A and its mixture with other extractants. Met Mater Int 21(1):166–172
Nayl AA, El-Nadi YA, Daoud JA (2009) Extraction and separation of Zr(IV) and Hf(IV) from nitrate medium by some Cyanex extractants. Sep Sci Technol 44(12):2956–2970
Benadict Rakesh K, Suresh A, Sivaraman N, Aswal VK, Vasudeva Rao PR (2016) Extraction and third phase formation behaviour of tri-iso-amyl phosphate and tri-n-butyl phosphate with Zr(IV) and Hf(IV): a comparative study. J Radioanal Nucl Chem 309(3):1037–1048
Taghizadeh M, Ghanadi M, Zolfonoun E (2011) Separation of zirconium and hafnium by solvent extraction using mixture of TBP and Cyanex 923. J Nucl Mater 412(3):334–337
Banda R, Lee MS (2015) Solvent extraction for the separation of Zr and Hf from aqueous solutions. Sep Purif Rev 44(3):199–215
Xu ZG, Zhao J, Wang LJ, Xu YL, Chi RA, Li PH, Jin X (2016) Kinetics for extraction of zirconium and hafnium in DIBK-P350 system. J Radioanal Nucl Chem 309(2):701–707
Mishra PK, Chakravortty V, Dash KC, Das NR, Bhattacharyya SN (1989) Extraction of zirconium(IV) from HCl solutions by mixtures of Aliquat-336 and Alamine-336 with TBP. J Radioanal Nucl Chem 134(2):259–264
Banda R, Lee HY, Lee MS (2013) Separation of Zr from Hf in acidic chloride solutions by using TOPO and its mixture with other extractants. J Radioanal Nucl Chem 298(1):259–264
Reddy BR, Kumar JR, Reddy AV (2004) Solvent extraction of zirconium(IV) from acidic chloride solutions using the thiosubstituted organophosphorus acids Cyanex 301 and 302. J Chem Technol Biotechnol 79(11):1301–1307
Reddy BR, Kumar JR, Raja KP, Reddy AV (2004) Solvent extraction of Hf(IV) from acidic chloride solutions using Cyanex 302. Miner Eng 17(7–8):939–942
Lee HY, Kim SG, Oh JK (2004) Stoichiometric relation for extraction of zirconium and hafnium from acidic chloride solutions with Versatic acid 10. Hydrometallurgy 73(1–2):91–97
Banda R, Lee HY, Lee MS (2012) Separation of Zr from Hf in hydrochloric acid solution using amine-based extractants. Ind Eng Chem Res 51(28):9652–9660
Wang LY, Lee HY, Lee MS (2015) Solvent extractive separation of zirconium and hafnium from hydrochloric acid solutions by organophosphorous extractants and their mixtures with other types of extractants. Chem Eng Commun 202(10):1289–1295
Saleh AS (2012) Solvent extraction of Zr(IV) and Hf(IV) with N, N, N′-N′-tetraoctyldiglycolamide. J Radioanal Nucl Chem 292(3):1109–1114
Xu ZG, Wang LJ, Wu YK, Chi RA, Zhang L, Wu M (2012) Solvent extraction of hafnium from thiocyanic acid medium in DIBK-TBP mixed system. Trans Nonferrous Met Soc China 22(7):1760–1765
Krishna GG, Reddy RS, Raghunath P, Bhanuprakash K, Kantam ML, Choudary BM (2004) A computational study of ligand interactions with hafnium and zirconium metal complexes in the liquid-liquid extraction process. J Phys Chem B 108(19):6112–6120
Chen S, Zhang ZF, Kuang ST, Li YL, Huang XW, Liao WP (2017) Separation of zirconium from hafnium in sulfate medium using solvent extraction with a new reagent BEAP. Hydrometallurgy 169:607–611
Lee MS, Banda R, Min SH (2015) Separation of Hf(IV)–Zr(IV) in H2SO4 solutions using solvent extraction with D2EHPA or Cyanex 272 at different reagent and metal ion concentrations. Hydrometallurgy 152:84–90
Reddy BR, Kumar JR, Reddy AV (2004) Solvent extraction of tetravalent hafnium from acidic chloride solutions using 2-ethyl hexyl phosphonic acid mono-2-ethyl hexyl ester(PC-88A). Miner Eng 17(4):553–556
Conradie EW, Van Der Westhuizen DJ, Nel JT, Krieg HM (2018) The hafnium-selective extraction fom a zirconium(hafnium) heptafluoride ammonium solution using organophosphorus-based extractants. Solvent Extr Ion Exch 36(7):658–673
Vermaak V, Krieg HM, De Beer L, Van der Westhuizen D (2018) Mechanistic study of hafnium and zirconium extraction with organophosphorus extractants. Solvent Extr Ion Exch 36(2):150–161
Das NR, Nandi B, Bhattacharyya SN (1981) Sequential separation of hafnium, zirconium and niobium from sulphuric acid medium using di(2-ethylhexyl)phosphoric acid as an extractant. Int J Appl Radiat Isot 32(4):205–209
Ryabchikov DI, Marov IN, Ermakov AN, Belyaeva VK (1964) Stability of some inorganic and organic complex compounds of zirconium and hafnium. J Inorg Nucl Chem 26(6):965–980
Singhal A, Toth LM, Lin JS, Affholter K (1996) Zirconium(IV) tetramer/octamer hydrolysis equilibrium in aqueous hydrochloric acid solution. J Am Chem Soc 118(46):11529–11534
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21876062) and the Natural Science Foundation of Shandong Province (ZR2017LB005).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, J., Yang, T., Zhang, H. et al. Preferential extraction of hafnium over zirconium with D2EHPA through selective complexation of organic acids. J Radioanal Nucl Chem 321, 333–339 (2019). https://doi.org/10.1007/s10967-019-06585-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06585-8