Abstract
Herein, a practical and simple reduction method was used to prepared the nanoscale composites of hydroxyapatite (HAP) coated with zero valent iron for uranium (U) efficient removal. The effects of the ratio of HAP, the dosage of the composites, pH value, contact time, initial concentration of U, temperature, and ions concentration of Mn2+ and CO32− on U adsorption were investigated, respectively. The maximum removal amount of the composites amounted to 155.8 mg g−1 when the initial pH was 4.0, temperature 298 K, and contact time 150 min. The removal process was analyzed by isotherms, kinetics, and thermodynamics. The relationship between adsorption capacity qe and equilibrium concentration Ce could be well described by a pseudo-second-order, intra-particle diffusion model and Langmuir isotherms. The synthesized composite is promising for efficiently treating U-contaminated groundwater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As a distinguished new energy source, nuclear power has recently received attention for its ability to satisfy basic energy requirements and relieve energy pressures [1,2,3]. Milling, refinement, disposal, agriculture sites, and accidents from nuclear power contribute to the increase in the concentration of uranium (U) in surface water, soil, and groundwater [4, 5]. Owing to its radioactivity and toxicity [6], U can eventually be a potential carcinogenic for humans causing kidney or liver damage, and it can accumulate in bones through the food chain. As an important component of water resources, even in the ecosystem, groundwater plays a key role in the water cycle [7]. Consequently, the removal of U from groundwater is of tremendous significance for environmental protection and human health.
Compared with other treatment technologies, permeable reactive barrier (PRB), a passive permeable treatment wall filled with reactive materials and installed in the path of a contaminated plume, has become more efficient, economical, and competitive for contaminated groundwater remediation. The effectiveness of a PRB highly depends on the reactive filling materials through their degradation, precipitation, and sorption processes due to the physical, chemical, biochemical, or integrated interactions [8]. Commonly used reactive materials include zero valent iron (ZVI), activated carbon (AC), phosphates, and clay minerals [9,10,11,12].
ZVI is the most widely utilized material in both laboratory studies and field applications [13, 14]. Because of its high reduction potential and large specific surface area, ZVI can activate various useful reaction mechanisms (e.g., redox reactions, precipitation, and sorption) for contaminant removal as a green in situ material. However, the use of ZVI has limitations in the long-term removal efficiency and hydraulic properties owing to the corrosive passivation and clogging of the barrier pores. In particular, anaerobic iron corrosion increases the pH and promotes the precipitation of secondary minerals, which have detrimental effects on the PRB longevity [15, 16]. However, the use of mixtures of iron with other reactive materials in various weight or volume ratios may assist in solving these problems. More than 60% of PRBs installed worldwide are reportedly iron-containing composites [17]. Using iron-containing materials has been considered as one of the most attractive and promising approaches for the removal of U from contaminated groundwater because they are highly efficient, inexpensive, and readily available [9, 11, 18, 19].
Hydroxyapatite (Ca10(PO4)6(OH)2, HAP) is a phosphate mineral (member of the apatite family). As a biomaterial (dense and porous bioceramics, coatings for implants or powder for gap filling), HAP has shown excellent potential in the remediation of groundwater owing to its innocuity, low cost, availability, environment friendly nature, and rich presence of hydroxyls. It is also promising as a filling material because of its excellent ion exchange properties for different heavy metals and adsorption ability of complex organic materials [20]. However, its characteristics of low density and fine grain cause always it to float on the water surface, and limit its practical usage, particularly in PRB applications. This limitation can be minimized by the use of HAP composites. Compared to bare HAP, HAP composite can enhance mass transfer process and more efficiently adsorb ions or molecules of a solution.
Nanoscale composites of HAP coated by ZVI is of a large specific surface area and a excellent potential for removing heavy metals. The mechanism of heavy metals removed by nanoscale composites should include adsorption, reduction of ZVI, precipitation of phosphate and so on. Among them, adsorption is very important, especially in PRB system. Retardarce of U migrate by the adsorption of nanoscale composites makes the reduction of heavy metals by ZVI and the phosphate precipitation by surface supersaturation become possible. However, the synthesis and adsorption properties of this composites are rare reports [20, 21].
The objective of this investigation was to introduce nanoscale composites of HAP coated by ZVI as a practical material for the removal of the target contaminant of U present in groundwater. For this purpose, the composite was synthesized and characterized, and its adsorptive features with reference to the dependency on the pH, time, initial concentration of U, dosage of the reaction material, temperature, and Mn2+ and CO32− content were investigated. Moreover, the equilibrium isotherms, kinetics, and thermodynamic models were also discussed.
Materials and methods
Chemicals and the nanoscale composites of HAP coated by ZVI preparation
HAP, U3O8, PVP (Polyvinyl Pyrrolidone), NaBH4, and FeSO4·7H2O in the present experiments were purchased from Sinopharm Chemical Reagent Co. LTD, P. R. China.
The nano HAP–ZVI composite was synthesized with HAP, NaBH4, and FeSO4·7H2O via the reduction method at ambient temperature. A 3.825 g of FeSO4·7H2O was dissolved in deionized water, and then the pH was adjusted by 4 mol L−1 NaOH and HCl solution. HAP was added and fully stirred. NaBH4 (1.5 g) and NaBH4 (1.0 g) were added successively and well mixed. A black solid was precipitated. The synthesized composite was washed thrice by deionized water, then ethanol, and finally by acetone, and then it was dried in a vacuum drying oven and spared [21, 22].
Solutions with various U concentrations were prepared via successive dilutions of the stock solution. The standard U stock solution of 0.5 g L−1 was prepared by dissolving the appropriate amount of U3O8 in deionized water and adding some drops of HNO3. All of the chemicals were of analytical reagent grade, and all the solutions were prepared with deionized water throughout the experiment.
Characterization of the composite
The structure and crystallinity of the as-prepared samples were characterized via X-ray diffraction (XRD) (D8 ADVANCE XRD, Bruker AXS) at 40 kV and 40 mA with high-intensity Cu-Kα radiation. The morphologies of the samples were examined using a scanning electron microscope (SEM) (Nova Nano 450, FEI) and transmission electron microscopy TEM (Tecnai-G20, FEI). The specific surface area was measured by an N2 adsorption apparatus (tristar IIPlus, Micromeritics Instrument Corp.). The functional groups were determined via Fourier transform infra-red (FTIR) spectra (Nicolet-460, Thermo Fisher Scientific). An electron spectrometer (Escalab 250Xi, Thermo Fisher Scientific) was employed to determine the surface functional groups with an Al-Kα X-ray source.
Experimental procedure
The removal of U by the nanoscale composites of HAP coated by ZVI was performed using the standard batch technique in an open laboratory. A certain concentration of U solution was incubated with the composite at various pH values (adjusted with HCl or NaOH solutions) and allowed to equilibrate while being continuously shaken at 140 min−1 in a vapor-bathing constant-temperature vibrator (ZD-85A, Shanghai Xiyuan Scientific Instrument Co., Ltd.). The supernatant liquid was analyzed after centrifugation. The concentration of U was determined (Agilent 5100 ICP-OES, Agilent Technologies). The removal rate (P%), and adsorption capacity (qe mg g−1) were calculated by the following formulas [23]:
where C0 and Ce are the initial and equilibrium concentrations of the U solution (mg L−1), respectively, V is the solution volume (mL), and m is the dosage of an adsorbent (mg).
Results and discussion
Characterization
In the present study, BET, SEM, TEM, XRD, FTIR, and XPS analyses were conducted to observe or measure the specific surface area, surface morphologies, structure, and element composition of the material.
The specific surface area was determined according to the standard Brunauer–Emmett–Teller (BET) method, while the average pore diameter was calculated directly from the N2 isotherm by applying the Barrett–Joyner–Halenda (BJH) method. The HAP and composite exhibit meso-porosity (Table 1) in reference to the IUPAC classification because the values of the pore diameters are 2–50 nm [24]. The surface area and porosity values of the composite are significantly higher than those of HAP. The N2 adsorption–desorption isotherms and curves of pore size distributions are presented in Fig. 1. The change in the physical characteristics of the composite is consistent with the different associated morphologies, as depicted in Fig. 2.
The morphology and nanostructures of the composites were characterized by SEM and TEM images. As shown in Fig. 2, the composite is crystalline, and its structure is similar to that of HAP. It exhibits a coarse poriferous surface and a lax structure. Moreover, it exhibits obvious agglomeration because the iron substitution can increase the crystallinity [25]. ZVI nanoparticles are highly polydispersed, ranging over tens to hundreds of nanometers in size. They display a gray edge and dark center, which demonstrate the presence of the typical metallic Fe0 core and iron oxide shell structure. Most of the Fe0 grains are loaded on the surface and pores of the HAP [26,27,28].
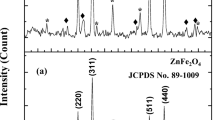
XRD spectra characteristics of the composite before and after U adsorption were shown in Fig. 3A. The composite is consistent with the 2θ diffractions at 25.88° (002), 31.77° (211), 32.90° (300), 44.67° (110), 49.41° (102) and 63.78° (200), when the scanning diffraction angle was in the range of 10°–70°. Compared with the standard JCPDF card, and the major peak is at 31.77°, which clearly confirms the presence of a well-crystallized HAP phase and proves the decomposition of CaO to form apatite [20, 29]. The appearance of typical peak for 44.67 confirms the presence of ZVI, and less iron oxide (FeOOH) is generated on the surface of ZVI. In addition, ZVI is proven to have a crystalline structure [30]. There was no visible difference in the position after U adsorption, but the intensity of these peaks were weeken differently.
The FTIR spectra of the composite before and after the reaction are compared in Fig. 3B. The strong and wide peaks at 3431 cm−1 are attributed to the strong vibration of the –OH groups of the adsorbed water [31]. The peaks at 603 and 564 cm−1 correspond to the P–O spreading vibration mode of the phosphate group. The peaks at 962 and 1036 cm−1 corresponded to its symmetric vibration, which reveal the strongest peaks in the PO4 vibrations [32,33,34]. After the adsorption, a new peak emerges at 881 cm−1, which is a characteristic peak of the uranyl ion group adsorbed by the composite [35,36,37]. The precipitate formed in the adsorbing course might have been autunite (Ca[UO2PO4]2·6H2O) or andersonite (Na2Ca(UO2)(–CO3)3·6H2O) [38]. There is no other great peak changes, which showed that the structure of composite did not change significantly.
The XPS spectra of the nanoscale composites are displayed in Fig. 4. The composite shows a clear presence of Fe, Ca, P, and O. The 2p3/2 peaks and 2p1/2 peaks of ZVI are at around 707 and 720 eV, respectively. The 2p3/2 peaks of oxidized iron and the 2p1/2 peaks (shake-up satellite) are also observed nearby 717 eV. The peaks indicate that the surface of Fe0 is covered with a layer of iron oxides (FeOOH or Fe2O3) [39, 40], which probably are due to the oxidation of the Fe0 exposed to air. After adsorption, the Fe0 peaks are not distinguished in the XPS spectrum of Fig. 4c [41]. The characteristic peak of U appears at the binding energy of 382.58 eV (Fig. 4d), which demonstrates that U is absorbed by the composite via chemical interactions [37, 42].
Effect of ratio and dosage
The composites were prepared via the reduction method with 3.825 g FeSO4·7H2O and 2 g, 3 g, 4 g, 5 g, 6 g, and 7 g HAP, respectively. The effect of the ratio of FeSO4·7H2O and the dosage of the composites on the removal of U at a contact time of 100 min was studied in a 10 mg L−1 U solution. The result shows that 3.825 g FeSO4·7H2O and 4 g HAP form the best ratio, as shown in Fig. 5a.
The relationship between the U removal and composite dosage is depicted in Fig. 5b. It can be seen that the sharp increase in the removal rate (74–95%) with the composite added from 0.005 to 0.020 g. However, the adsorption capacity decrease (27–150 mg g−1) rapidly. The higher removal rate could be attributed to the increase in the number of adsorption sites with the increasing amount of the composite. The decrease of adsorption capacity for U may be explained with the increase in the total surface area and diffusion path length of U with increasing dosage [12, 43]. Then the removal rate and the adsorption capacity maintain stable after equilibrium. The optimum dosage of the composite is 0.2 g L−1 in this system.
Effect of pHinitial
The effect of a pH on the U removal was investigated over a pH range from 2.0 to 8.0 at 25 °C for 100 min using 10 mg L−1 as the initial U concentration. The results are displayed in Fig. 6. The pH has a high impact on the removal of U, which may affect the presence of U and active sites of the composite. The initial removal rate significantly increase from 2.0 to 4.0, reaching maximum removal of U at a pHinitial value of 4 and then gradually decreasing. U mainly exists in the form of UO22+. The H+ ions will compete with UO22+ for the active sites, and ZVI will react with H+ to generate Fe2+ at a lower pH, affecting the U reduction and precipitation. Then U can be reduced easily by ZVI on the surface of the composite under weakly acidic conditions, with UO22+ forming the insoluble UO2. Under near neutral or alkaline conditions, uranium mainly exists in the form of a uranyl hydroxy complex and polynuclear hydroxy uranyl complex UO2(OH)n ((UO2(OH)+, (UO2)2(OH)22+, (UO2)3(OH)5+, (UO2)4(OH)7+, (UO2)3(OH)7)) [44], which have larger ionic radii and lower adsorption affinities than those of UO22+, become the dominating species, resulting in a decline in qe [45, 46]. The presence of these complexes will inhibit the adsorption, reduction, and precipitation of U.
Effect of time and initial U concentration
As shown in Fig. 7a, the adsorption capacity of U increase with prolonged time. The initial sorption is very rapid owing to the lower mass transfer resistance on the surface with the continuation of sorption. Then, restricted by the number of adsorption sites and corrosion products (iron oxide) on the surface of the composite, the qe increases slowly, and maintain stability after 150 min. Therefore, in all the subsequent experiments, we selected 150 min as the contact time to ensure the reaction equilibrium was attained.
Figure 7a shows the effect of the initial U concentration on the adsorption capacity of the composite. The removal of U is highly concentration dependent. The initial concentration increase from 5 to 40 mg L−1, and the adsorption capacity increase from 23.2 to 155.8 mg g−1. The increases of uranyl ions amount provides an important driving force for overcoming the U mass transfer limitations between the solid and aqueous phases. For all further studies, the concentration was selected as 10 mg L−1. The Fe concentration variation is displayed in Fig. 7b. It is obvious that the Fe concentration is too low to cause secondary pollution.
Kinetic and sorption isotherms
Modeling of the sorption kinetics
In order to clarify and understand the sorption mechanisms and rate-limiting step, the five kinetic models (the pseudo-first-order, pseudo-second-order, Elovich model, film diffusion and intraparticle diffusion models are used to investigate the controlling mecheanism of the U adsorption process by the composite [47,48,49]. The parameters of the sorption kinetic models are tabulated in Table 2, and the curves of fitting models are exhibited in Fig. 8.
It could be clearly observed that the U sorption by the composite is comparatively suited to the pseudo-second-order kinetic model, which also assumes that the rate-limiting step may be a chemisorption process involving valence forces through the sharing or exchanging of electrons between U and the surface sites of composite. The Elovich equation is employed successfully to describe the second-order kinetics assuming that the composite surfaces are energetically heterogeneous. Much higher value of α than β indicates a higher adsorption rate than the desorption rate, which exhibits the viability of the adsorption process.
Straight lines do not pass through the origin, which suggests that the film and intra-particle diffusion are the rate-limiting steps. The value of the intercept of the film diffusion is very close to zero suggesting that the sorption kinetics of the composite may be controlled by the diffusion through the liquid film surrounding it. However, because the intercept is not exactly zero and a higher value of correlation coefficient (R2) indicates a good fitness of the intra-particle diffusion model. It is concluded that the adsorption kinetics may be simultaneously controlled by the film diffusion and intra-particle diffusion.
Modeling of the sorption isotherms
Several mathematical models can be used to describe the experimental data of sorption isotherms. The Langmuir, Freundlich, Tempkin, and Dubinin–Radushkevich models were adopted to simulate the sorption isotherms. This work aims to specify the parameters of the models, compare the behavior of the U sorption, and determine the theoretical sorption isotherms [50, 51]. The fitting results for the U removal by the composite are shown in Fig. 9, and the calculated constants are listed in Table 3. Correlation coefficient R2 of the Langmuir adsorption isotherm model was larger than that for the other sorption isotherm models. Therefore, the removal of U by the composite is effectively described by the Langmuir isotherm model, indicating that the sorption is primarily a monolayer process.
Dimensionless constant separation factor RL [52, 53] is listed in Table 4. The RL values are in the range of 0 < RL < 1, which indicates that the removal of U by the composite is favorable in the concentration range used in this study. Accordingly, the composite is a favorable adsorbent. The 1/nf value (0.497) shows that it is easy to adsorb. Furthermore, the bT value (defined as the variation in the adsorption energy in the Temkin model) of 68.197 suggests that the U adsorption on the composite surface occurs exothermically (bT > 1) because of the electrostatic interaction and heterogeneous pores. The energy value (1.827 kJ mol−1) of the Dubinin–Radushkevich model is lower than 8 kJ mol−1, indicating that the sorption process of U on the composite is dominated by physisorption.
Effect of temperature and sorption thermodynamics
The effect of the temperature on the uranium sorption on the composite is depicted in Fig. 10a. The values of adsorption capacity and removal rate slightly increase with temperature increment, which suggests that higher temperatures are beneficial for the U removal process. Therefore, the temperature has some impact, with the increase in the adsorption capacity being probably because of appearance of some new adsorption sites on the surface of the composite under the effect of the heat activation [54].
The thermodynamic parameters such as the change in the entropy (ΔS0), enthalpy (ΔH0), and Gibbs free energy (ΔG0) can be calculated using the following equations, and their fitting results are presented in Fig. 10b and Table 5.
where Kc is the distribution coefficient. The slope and intercept of the linearized plot of ln Kc versus 1/T are used to calculate ΔH0 and ΔS0.
The U removal performance by the composite is spontaneous (ΔG0 < 0), endothermic and the randomness (ΔH0 > 0, ΔS0 > 0) at the solid and solution interface. Moreover, the decrease in ΔG0 with increasing temperature shows that the process is favorable to higher temperatures.
Effect of manganese and carbonate ion concentration
Manganese (Mn) is widely distributed, and its dissolved state is mainly Mn2+, CO32− and HCO3− ions are also common, in the groundwater in our country. The study to reveal effect of ionic intensity on sorption of U in the presence of MnCl2 or Na2CO3 indicated that the presence of Mn2+ and CO32− had different effect on U removal. The removal rate of U decreases with increasing Mn2+ and CO32− concentration from 0 to 0.2 mg L−1. The amount of U sorption changes slightly by Mn2+ because there may be two different ways and sites of adsorption for the adsorption of Mn and U. However, the removal rate decreases sharply with increasing CO32− (see Fig. 11). In view of the omnipresence of carbonates in groundwater, such U-carbonato surface complexation plays a significant role in the retention of U in the contaminated sites [55].
Mechanism analysis of U sorption
Four mechanisms for U sorption by the nano HAP–ZVI composite, which slightly differed from the system HAP and ZVI due to the formation of composite: (1) the partial dissolution of HAP under acidic conditions yields a small amount of Ca2+ and PO43− that precipitates as H2[(UO2)(PO4)]2·nH2O or Ca[(UO2)(PO4)]2·nH2O [4, 56]. (2) Fe0 can efficiently provide electrons for U reduction [57, 58]. (3) The Ca2+ surface sites can react with U via ion exchange (isomorphous substitutions) [20, 59]. (4) The UO22+ occupies the actively adsorptive sites of ≡OH or ≡O3P–OH+ to release H+ [60].
Conclusions
In this work, the effects on U removal by the nano HAP–ZVI composite were investigated using batch sorption experiments. When the dosage was 0.02 g, initial concentration of U was 10 mg L−1, pH was 4, temperature was 25 °C, and time was 100 min, the removal ratio was 93.8% and adsorption capacity was 46.9 mg g−1. The XRD analysis showed that most particles on the composite were Fe0 and a small amount of iron oxide (FeOOH) was covered on the surface of Fe0. The SEM analysis revealed that the size of the composite particles was more uniform and that Fe0 particles were present on the surface and in the pores of the composite.
The removal process of U on the composite was in line with the pseudo second-order kinetic model and Langmuir adsorption isotherm model. The adsorption kinetics might be controlled by the film diffusion and intra-particle diffusion simultaneously. Thermodynamic studies showed that the process was chemical sorption and endothermic and spontaneous. The U removal capacity of the composite was significantly influenced by the CO32− concentration. More CO32− increased the pH, thereby influencing the active sites of the composite and presence of U.
In summary, our study highlighted the removal of U from groundwater using the nano HAP–ZVI composite, which is highly applicable for removing U species from water in natural environments. The composite is a potential material that can be used for removal of U.
References
Gu PC, Zhang S, Li X, Wang XX, Wen T, Riffat J, Ahmed A, Tasawar H, Wang XK (2018) Recent advances in layered double hydroxide-based nanomaterials for the removal of radionuclides from aqueous solution. Environ Pollut 240:493–505
Zhao GX, Huang XB, Tang ZW, Huang QF, Niu FL, Wang XK (2013) Polymer-based nanocomposites for heavy metal ions removal from aqueous solution: a review. Polym Chem 9:3562–3582
Li J, Wang XX, Zhao GX, Chen CL, Chai ZF, Ahmed A, Tasawar H, Wang XX (2018) Metal-organic framework-based materials: superior adsorbents for the capture of toxic and radioactive metal ions. Chem Soc Rev 47:2322–2356
Liu J, Zhao CS, Zhang ZB, Liao JL, Liu YH, Cao XH, Yang JJ, Yang YY, Liu N (2016) Fluorine effects on U(VI) sorption by hydroxyapatite. Chem Eng J 288:505–515
Chen A, Shang C, Shao J, Zhang J, Huang H (2017) The application of iron-based technologies in uranium remediation: a review. Sci Total Environ J 575:1291–1306
Li SN, Bai HB, Wang J, Jing XY, Liu Q, Zhang ML, Chen RR, Liu LH, Jiao CS (2012) In situ grown of nano-hydroxyapatite on magnetic CaAl-layered double hydroxides and its application in uranium removal. Chem Eng J 193–194:372–380
Liu YY, Mou HY, Chen LQ, Zakaria AM, Liu L (2015) Cr(VI)-contaminated groundwater remediation with simulated permeable reactive barrier (PRB) filled with natural pyrite as reactive material: environmental factors and effectiveness. J Hazard Mater 298:83–90
Thiruvenkatachari R, Vigneswaran S, Naidu R (2008) Permeable reactive barrier for groundwater remediation. J Ind Eng Chem 14:145–156
Zhou D, Li Y, Zhang YB, Zhang C, Li XF, Chen ZL, Huang JY, Li X, Giancarlo F, Masashi K (2014) Column test-based optimization of the permeable reactive barrier (PRB) technique for remediating groundwater contaminated by landfill leachates. J Contam Hydrol 168:1–6
Zhang ZX, Liu HB, Lu P, Chen TH, Ma WJ (2018) Nanostructured α-Fe2O3 derived from siderite as an effective Hg(II) adsorbent: Performance and mechanism. Appl Geochem 96:92–99
Richard A, Crane S (2014) The removal of uranium onto carbon-supported nanoscale zero-valent iron particles. J Nanopart Res 16:2813
Guo Yd, Liu YY (2014) Adsorption properties of methylene blue from aqueous solution onto thermal modified rectorite. J Dispers Sci Technol 35:1351–1359
Faisal AAH, Sulaymon AH, Khaliefa QM (2018) A review of permeable reactive barrier as passive sustainable technology for groundwater remediation. Int J Environ Sci Technol 15:1123–1138
Lu X, Li M, Deng H, Lin PF, Mark RM, Liu X (2016) Application of electrochemical depassivation in PRB systems to recovery Fe0 reactivity. Front Environ Sci Eng 10:04
Ruhl AS, Martin J (2012) Impacts of Fe0 grain sizes and grain size distributions in permeable reactive barriers. Chem Eng J 213:245–250
Carniato L, Schoups G, Seuntjens P, Van NT, Simons Q, Bastiaens L (2012) Predicting longevity of iron permeable reactive barriers using multiple iron deactivation models. J Contam Hydrol 142:93–108
Gyanendra R, Buddhima I, Long DN (2009) Long-term performance of a permeable reactive barrier in acid sulphate soil terrain. Water Air Soil Pollut 9:409
Stepanka K, Miroslav C, Lenka L, Jan F, Dalibor J, Dalibor J, Zboril Radek (2011) Zero-valent iron nanoparticles in treatment of acid mine water from in situ uranium leaching. Chemosphere 82:1178–1184
Mueller NC, Jürgen B, Johannes B, Če Miroslav, Peter R, David R, Bernd N (2012) Application of nanoscale zero valent iron (NZVI) for groundwater remediation in Europe. Environ Sci Pollut Res 19:550–558
Demet B, Ulvi U (2012) Polyacrylamide–hydroxyapatite composite: preparation, characterization and adsorptive features for uranium and thorium. J Solid State Chem 194:1–8
Liu W, Tian ST, Zhao X, Xie WB, Gong YY, Zhao DY (2015) Application of stabilized nanoparticles for in situ remediation. Curr Pollut Rep 1:280–291
Liu DQ, Liu ZR, Wang CF, Lai Y (2016) Removal of uranium(VI) from aqueous solution using nanoscale zero-valent iron supported on activated charcoal. J Radioanal Nucl Chem 310:1131–1137
Wang XX, Yu SQ, Wu YH, Pang HW, Yu SJ, Chen ZS, Hou J, Ahmed A, Tasawar H, Wang SH (2018) The synergistic elimination of uranium (VI) species from aqueous solution using bi-functional nanocomposite of carbon sphere and layered double hydroxide. Chem Eng J 342:321–330
Yang TR (2003) Adsorbents: fundamentals and applications. Wiley, Canada
Hae-Won K, Li LH, Koh YH, Jonathan CK, Hyoun-Ee K (2004) Sol–gel preparation and properties of fluoride-substituted hydroxyapatite powders. J Am Ceram Soc 87:1939–1944
Hu BW, Mei X, Li X, Hu J, Xu D, Ma JY, Huang YY (2017) Decontamination of U(VI) from nZVI/CNF composites investigated by batch, spectroscopic and modeling techniques. J Mol Liq 237:1–9
Cheng WC, Ding CC, Wang XX, Wu ZY, Sun YB, Yu SH, Tasawar H, Wang XK (2016) Competitive sorption of As(V) and Cr(VI) on carbonaceous nanofibers. Chem Eng J 293:311–318
Ding CC, Cheng WC, Sun YB, Wang XK (2015) Effects of Bacillus subtilis on the reduction of U(VI) by nano-Fe0. Geochim Cosmochim Acta 165:86–107
Bajpai AK, Bundela H (2009) Development of poly(acrylamide)-hydroxyapatite composites as bone substitutes: Study of mechanical and blood compatible behavior. Polym Compos 30:1532–1543
Richard AC, Thomas S (2014) The removal of uranium onto carbon-supported nanoscale zero-valent iron particles. J Nanoparticle Res 16:1–13
Sudip M, Biswanath M, Apurba D, Sudit SM (2012) Studies on processing and characterization of hydroxyapatite biomaterials from different biowastes. J Miner Mater Charact Eng 11:55–67
Li FB, Li XY, Cui P (2018) Detoxification of U(VI) by Paecilomyces catenlannulatus investigated by batch, XANES and EXAFS techniques. J Environ Radioact 189:24–30
Sudip M, Giang H, Panchanathan M, Madhappan SM, Thanh PN, TuongVP Thi, Hye HK, Myoung HK, Seung YN, Junghwan O (2018) Nano-hydroxyapatite bioactive glass composite scaffold with enhanced mechanical and biological performance for tissue engineering application. Ceram Int 44:15735–15746
Hyehyun K, Sudip M, Bian J, Panchanathan M, Madhappan SM, Junghwan O (2018) Biomimetic synthesis of metal–hydroxyapatite (Au-HAp, Ag-HAp, Au-Ag-HAp): Structural analysis, spectroscopic characterization and biomedical application. Ceram Int 44:20490–20500
Liu MX, Dong FQ, Yan XY, Zeng WM, Hou LY, Pang XF (2010) Biosorption of uranium by Saccharomyces cerevisiae and surface interactions under culture conditions. Bioresour Technol 101:8573–8580
Li L, Ding DX, Hu N, Fu PK, Xin X, Wang YD (2014) Adsorption of U(VI) ions from low concentration uranium solution by thermally activated sodium feldspar. J Radioanal Nucl Chem 299:681–690
Wang BD, Zhou YX, Li L, Wang Y (2018) Preparation of amidoxime-functionalized mesoporous silica nanospheres (ami-MSN) from coal fly ash for the removal of U(VI). J Sci Total Environ 626:219–227
Agnieszka GP, Ewelina G, Marek M (2018) Simultaneous adsorption of uranium (VI) and phosphate on red clay. J Nucl Energy 104:150–159
Xiao JA, Gao BY, Yue QY, Gao Y, Li Q (2015) Removal of trihalomethanes from reclaimed-water by original and modified nanoscale zero-valent iron: characterization, kinetics and mechanism. Chem Eng J 262:1226–1236
Wu YW, Yue QY, Ren ZF, Gao BY (2018) Immobilization of nanoscale zero-valent iron particles (nZVI) with synthesized activated carbon for the adsorption and degradation of Chloramphenicol (CAP). J Mol Liq 262:19–28
Xi YF, Megharaj M, Ravendra N (2010) Reduction and adsorption of Pb2+ in aqueous solution by nano-zero-valent iron—a SEM, TEM and XPS study. Mater Res Bull 45:1361–1367
Scott TB, Tort OR, Allen GC (2007) Aqueous uptake of uranium onto pyrite surfaces;reactivity of fresh versus weathered material. Geochim Cosmochim Acta 71:5044–5053
Tan XL, Fang M, Tan LQ, Liu HN, Ye XS, Tasawar H, Wang XK (2018) Core–shell hierarchical C@Na2Ti3O7·9H2O nanostructures for the efficient removal of radionuclides. Environ Sci Nano 5:1140–1149
Qian LP, Ma MH, Cheng DH (2015) Adsorption and desorption of uranium on nano goethite and nano alumina. J Radioanal Nucl Chem 303:161–170
Li XL, Wu JJ, Liao JL, Zhang D, Yang JJ, Feng Y, Zeng JH, Wen W, Yang YY, Tang J (2013) Adsorption and desorption of uranium (VI) in aerated zone soil. J Environ Radioact 115:143–150
Ahmed MD, Asem AA, Ewais MM, Anas ME, Mahmoud OE (2009) Removal of uranium (VI) from aqueous solutions using glycidyl methacrylate chelating resins. Hydrometallurgy 95:183–189
Hyun MJ, Seunghyun Y, Yong-Keun C, Sunkyu P, Eunsung K (2018) Adsorption isotherm, kinetic modeling and mechanism of tetracycline on Pinus taeda-derived activated biochar. Bioresour Technol 259:24–31
Velazquez-Peña Guadalupe C, Solache-Ríos M, Olguina MT, Fall Cheikh (2019) As(V) sorption by different natural zeolite frameworks modified with Fe, Zr and FeZr. Microporous Mesoporous Mater 273:133–141
Jafar A, Niyaz MM, Manouchehr V, Iran A (2019) Synthesis of magnetic metal-organic framework nanocomposite (ZIF-8@SiO2@MnFe2O4) as a novel adsorbent for selective dye removal from multicomponent systems. Microporous Mesoporous Mater 273:177–188
Ahamad KU, Singh R, Baruah I, Choudhury H, Sharma MR (2018) Equilibrium and kinetics modeling of fluoride adsorption onto activated alumina, alum and brick powder. J Groundw Sustain Dev 7:452–458
Zhang ZB, Dong ZM, Wang XX, Ying D, Niu FL, Cao XH, Wang YQ, Hua R, Liu YH, Wang XK (2018) Ordered mesoporous polymer–carbon composites containing amidoxime groups for uranium removal from aqueous solutions. Chem Eng J 341:208–217
Wang GH, Liu JS, Wang XG, Xie ZY, Deng NS (2009) Adsorption of uranium (VI) from aqueous solution onto cross-linked chitosan. J Hazard Mater 168:1053–1058
Jiménez-Reyes M, Solache-Ríos M (2010) Sorption behavior of fluoride ions from aqueous solutions by hydroxyapatite. J Hazard Mater 180:297–302
Zareh MM, Aldaher A, Hussein AEM, Mahfouz MG, Soliman M (2013) Uranium adsorption from a liquid waste using thermally and chemically modified bentonite. J Radioanal Nucl Chem 295:1153–1159
Yongheum J, Jun-Yeop L, Jong-Il Y (2018) Adsorption of uranyl tricarbonate and calcium uranyl carbonate onto γ-alumina. J Appl Geochem 94:28–34
Chen Z, Chen WY, Jia DS, Liu Y, Zhang AR, Wen T, Liu J, Ai YJ, Song WG, Wang XK (2018) N, P, and S codoped graphene-like carbon nanosheets for ultrafast uranium (VI) capture with high capacity. Adv Sci 5:1800235
Lv ZM, Yang SM, Chen L, Ahmed A, Tasawar H, Chen CL (2019) Nanoscale zero-valent iron/magnetite carbon omposites for highly efficient immobilization of U(VI). J Environ Sci 76:377–387
Song S, Yin L, Wang XX, Liu L, Huang SY, Zhang R, Wen T, Yu SJ, Fu D, Tasawar H, Wang XK (2018) Interaction of U(VI) with ternary layered double hydroxides by combined batch experiments and spectroscopy study. Chem Eng J 338:579–590
Sun YB, Lan JH, Li MX, Hu W, Liu HB, Song G, Chen DY, Shi WQ, Wang XK (2018) Influence of aqueous sulfide on speciation of U(VI) adsorbed to nanomagnetite. Environ Sci Nano 5:1981–1989
Yao W, Wang XX, Liang Y, Yu SJ, Gu PC, Sun YB, Xu C, Chen J, Tasawar H, Ahmed A, Wang XK (2018) Synthesis of novel flower-like layered double oxides/carbon dots nanocomposites for U(VI) and 241Am(III) efficient removal: batch and EXAFS studies. Chem Eng J 332:775–786
Acknowledgements
This work was financially supported by the National Natural Science Fund Program (41562011, 41502235), the Foundation for Educational Commission of Jiangxi Province of China (GJJ150577), the Fundamental Science on Radioactive Geology and Exploration Technology Laboratory (Z201406, RGET1509).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zeng, H., Lu, L., Gong, Z. et al. Nanoscale composites of hydroxyapatite coated with zero valent iron: preparation, characterization and uranium removal. J Radioanal Nucl Chem 320, 165–177 (2019). https://doi.org/10.1007/s10967-019-06451-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06451-7