Abstract
Radiochronometry analyses of two Pu metals were performed using the 237Np–241Am–241Pu, 234U–238Pu, 235U–239Pu, and 236U–240Pu decay series. For one sample, all radiochronometers yield concordant model dates in 1959–1960, indicating that the aqueous processing method used to purify Pu effectively removed U, Am and Np decay products from the bulk Pu. The second sample yields discordant model dates that are also older than the known production date in 1982. The excess U, Am and Np present in the sample indicate that the sample was purified during at least two different episodes, using a combination of aqueous methods and molten salt extraction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The central goal of a nuclear forensic investigation is to understand the history of an unknown sample as fully as possible, in part by identifying signatures that can constrain when and how a sample was made [1]. The radiometric model age of a sample of nuclear material is a powerful predictive signature of a material’s history, provided that key assumptions about the sample’s history are met. The core assumption that underlies a model age calculation is that the sample was fully purified of decay products at some point during the material’s production. For many forensic samples it is not possible to know this independently. However, applying multiple decay series chronometers to a sample provides a means to test the assumption that the material was fully purified of decay products at some time in the past. For example, concordant model ages determined using multiple daughter–parent or granddaughter–parent nuclide pairs provide a high degree of confidence that the material was highly pure at the time that it was produced, and also that the model ages represent that time of purification [e.g., 2–5]. When measured in an individual sample, the three U–Pu daughter–parent isotope pairs (234U–238Pu, 235U–239Pu, 236U–240Pu) and the 237Np–241Am–241Pu granddaughter–daughter–parent series that are commonly used in Pu radiochronometry provide the ability to test this assumption. These isotope series also provide an opportunity to evaluate purification methods, which produce signatures in the differential purification of the decay products, that may have been used to produce the plutonium sample.
With this study, we have measured actinide decay series radiochronometers in two samples of Pu metal that were produced in the United States by production methods that were in common use at the time. The two samples investigated were retrieved from storage at Lawrence Livermore National Laboratory. One sample, Metal-1, was known to be older, originating in the early 1960s. The other sample, Metal-2, was younger, and was made into its final form (from which it was later sub-sampled for radiochronometry analysis) on the known date of 24-August-1982. In the 1950s and 1960s, plutonium was commonly purified by aqueous processing methods, including solvent extraction and ion exchange, and then converted to metal by fluoride bomb reduction [6,7,8]. By the 1970s, additional purification methods such as molten salt extraction (MSE) and electrorefining (ER) had been developed and were in common use [6, 9,10,11]. Therefore, Metal-1 and Metal-2 samples were likely produced using different purification methods. The goal of this study is to evaluate the signatures of different plutonium production methods using multiple actinide decay series radiochronometers.
Analytical methods
Sample preparation and purification
All initial sample preparation, dissolution and dilution activities were performed in a nitrogen-atmosphere glovebox. Metal samples were cleaned by grinding and electropolishing (which removes surface oxide), weighed, and then dissolved in HCl. The starting mass of Metal-1 was 117.4 mg, and the starting mass of Metal-2 was 421.4 mg. Sample solutions were serially diluted with HNO3 and trace HF to obtain dilutions with a concentration of 20 \(\upmu\)g sample/g solution. Ten milliliter of each 20 ppm sample solution were transferred from the glovebox to a chemical fume hood suitable for handling lower levels of radioactivity, where subsequent sample preparation was performed. A sample digestion and dilution blank was prepared alongside the samples in the glovebox and transferred to the low-level chemical fume hood for analysis with the samples.
An aliquot of the 20 ppm sample solution was gravimetrically diluted to 10 ppb using 2 M HNO3 + 0.05 M HF, and aliquots of this dilution were used for Pu isotopic composition analysis, as well as Pu and Am concentration analysis by isotope dilution mass spectrometry (IDMS). Aliquots of the 20 ppm primary solution were used for U and Np analyses. Baseline® acids from Seastar Chemicals were used for sample preparation and purification. Columns for sample purifications were prepared using Bio-Rad Poly-Prep columns and AG 1-X8 100–200 mesh anion exchange resin (Bio-Rad), AG MP-1M 100–200 mesh anion exchange resin (Bio-Rad), TEVA resin (Eichrom Technologies) or UTEVA resin (Eichrom Technologies).
An aliquot of the 10 ppb dilution for each sample was weighed and spiked with a 242Pu tracer (NIST SRM 4334H) for concentration analysis by IDMS. An unspiked aliquot was also taken for Pu isotopic composition analysis by MC-ICP-MS and alpha spectrometry. Plutonium was purified from the spiked and unspiked aliquots using a two-column procedure. For the first column, Pu was dissolved in 8 M HNO3 + NaNO2 and loaded on a 1.4 mL AG 1-X8 anion resin bed. The column was rinsed with 8 M HNO3 and then 9 M HCl. Plutonium was eluted from the column using 9 M HCl + HI. For the second column, Pu was dissolved in 4 M HNO3 + NaNO2 and loaded on a 0.6 mL TEVA-resin bed. The column was rinsed with 4 M HNO3 and then 9 M HCl. The Pu was eluted in 9 M HCl, followed by 0.1 M HCl + 0.005 M HF and then 0.1 M HCl + HI.
Aliquots of the 10 ppb dilutions of the two samples containing approximately 30 ng of Pu were spiked with an 243Am tracer (NIST SRM 4332D) for Am concentration analysis by IDMS. Americium was purified using three stages of anion exchange chemistry. First, the sample was dissolved in 8 M HNO3 and loaded on a 1.4 mL AG 1-X8 anion resin bed, and Am was eluted with further rinses with 8 M HNO3. Next, the sample was dissolved in 9 M HCl + HNO3, and loaded on a 1.0 mL AG 1-X8 anion resin bed. The Am was eluted with further rinses with 9 M HCl. Last, Am was dissolved in a mixture of 3 parts acetone and 1 part concentrated HCl and loaded on a 1.0 mL AG 1-X8 anion resin bed. The column was rinsed in the acetone–HCl solution, and then Am was eluted in concentrated HCl.
An aliquot of each sample containing approximately 40 \(\upmu\)g of Pu was used for U isotopic composition analysis by MC-ICP-MS. Aliquots containing 10–20 \(\upmu\)g of Pu were spiked with a 233U tracer for U concentration analysis by IDMS. Uranium was separated from the bulk Pu sample using a LaF3 precipitation procedure wherein Pu is incorporated into a fluoride precipitate while U remains in the supernate. The supernate was decanted, dried, dissolved in 4 M HNO3, and loaded on a 0.6 mL TEVA resin bed. Uranium was eluted from the TEVA resin with further rinses with 4 M HNO3. The final purification of U was accomplished using a 0.6 mL UTEVA resin bed. The sample was dissolved in 4 M HNO3 and loaded on the column; the column was rinsed with 4 M HNO3, 9 M HCl and 5 M HCl, and then U was eluted in 0.1 M HCl.
Neptunium concentrations were determined by MC-ICP-MS using an external sensitivity calibration. An aliquot of each sample containing approximately 10 \(\upmu\)g of Pu was spiked with a 239Np radiochemical recovery tracer. The first purification was performed using a 1.5 mL bed of AG MP-1 anion resin. The sample was dissolved in a solution of 1 M HNO3-methanol-0.1 M hydroxylamine hydrochloride and loaded on the column. The resin bed was further rinsed with HNO3-methanol-hydroxylamine hydrochloride, and Np was eluted in 1 M HCl + 0.5 M HF. Neptunium was further purified using a 0.3 mL AG 1-X8 anion resin column. The sample was dissolved in 9 M HCl and loaded on the column. The resin bed was further rinsed with 9 M HCl and then Np was eluted in concentrated HBr. Neptunium recovery was determined through gamma-spectrometry measurement of the 239Np tracer in the sample compared with the unpurified tracer in the same geometry. Neptunium recoveries were > 95% for both samples, and the uncertainty associated with Np recovery is incorporated into the reported uncertainties for Np concentration.
Sample analysis by MC-ICP-MS and alpha-spectrometry
Plutonium isotopic analyses were performed using a Nu Plasma HR MC-ICP-MS, with the exception of 238Pu, which was measured by alpha-spectrometry. Plutonium analysis by MC-ICP-MS utilized a two-cycle analysis routine with a mixed collector array, with 239Pu and 240Pu measured on Faraday collectors and 240Pu, 241Pu, 242Pu and 244Pu measured on ion counters. Instrumental mass bias and detector gain corrections were applied using CRM 137 as a reference standard. Alpha-spectrometry analyses of 238Pu utilized Ortec ULTRA-AS ion-implanted Si detectors. The 238Pu/239Pu ratios were calculated using the background-subtracted integrals of peaks associated with 238Pu (5.499 and 5.456 MeV) and 240Pu + 239Pu (5.168, 5.124, 5.157, 5.144, 5.106 MeV), along with the 240Pu/239Pu determined by mass spectrometry. Corrections were made for decay during counting, although these were negligible. The Pu blank introduced during sample dissolution and purification was negligible relative to the sample aliquot size, and no blank correction was applied to Pu analytical results. Plutonium isotopic results are given in Table 1.
Uranium isotopic analyses were performed using a Nu Plasma HR MC-ICP-MS, using a static analysis routine with all uranium masses on Faraday collectors. Uranium mass bias corrections were made using CRM U010 as a reference standard. The uranium content and isotopic composition were measured for a single sample dissolution and dilution blank (from sample processing in the glovebox) as well as for two blanks representing the U chemical purification procedures. These blanks were measured using a mixed Faraday-ion counter analysis routine on a Nu Plasma II MC-ICP-MS. Total uranium amounts of approximately 19 ng (Metal-1) and 28 ng (Metal-2) were purified for analysis. The average blank was 246 ± 12 pg U with an isotopic composition as follows: 238U/235U = 50 ± 15, 236U/235U = 0.0141 ± 0.0066, 234U/235U = 0.0117 ± 0.0045 (stated uncertainties for blank values are combined standard uncertainties, k = 1). The U isotopic results have been corrected for this blank and the uncertainty associated with the blank correction has been incorporated into the reported uncertainties. The blank correction had a negligible effect on the 234U/235U and 236U/235U ratios. The effect on 238U/235U was substantial, due to the very low abundance of ingrown 238U in Pu, and the high relative abundance of 238U in the laboratory blank. Because of the large blank correction on 238U, analytical results for 238U are presented and discussed only in general terms. Uranium isotopic results are given in Table 2.
Americium isotopic analyses were completed using a Nu Plasma HR MC-ICP-MS, using a static multi-ion counting routine with 241Am and 243Am measured simultaneously on ion counters. Uranium CRM U010 was used as a reference standard for correction of instrumental mass bias and also relative detector gains. The Am blank introduced during sample dissolution and purification was below the detection limit of the analytical method, and no blank correction has been applied to the Am analytical results. Americium assay results are presented in Table 3.
Neptunium concentration analyses were completed using a Nu Plasma HR MC-ICP-MS. An external Np sensitivity curve was prepared using an in-house Np concentration standard. The in-house Np standard was gravimetrically prepared from Np metal for which the assay was determined as 100% − impurities, with the impurities quantified by ICP-MS. Neptunium concentration standards and purified Np samples were spiked with 233U as an internal sensitivity tracer. The Np blank introduced during sample dissolution and purification was below the detection limit of the analytical method, and no blank correction has been applied to the Np analytical results. Assay results are presented in Table 3.
Actinide isotopic compositions and concentrations
The two samples have similar Pu isotopic compositions. Metal-1 has a slightly lower 240Pu content (5.858 ± 0.074 at.%) than Metal-2 (5.911 ± 0.070 at.%), and the abundances of the other minor Pu isotopes are similarly lower in Metal-1 relative to Metal-2. 244Pu was not detected in either sample. The 234U/235U and 236U/235U ratios of both samples are consistent with the expected isotopic composition of uranium that has ingrown from decay of plutonium. Metal-1 has a higher relative abundance of 238U compared to Metal-2. Uranium isotope ratios have been corrected for the measured laboratory blank, so the elevated 238U in Metal-1 likely reflects a higher proportion of natural uranium contamination that was introduced to this sample some time before destructive analysis began. The potential origin of this excess uranium is discussed in more detail in the following section.
The samples have Pu assay values of 0.9702 ± 0.0089 g Pu/g sample and 0.9784 ± 0.0083 g Pu/g sample for Metal-1 and Metal-2, respectively. The U concentrations are 1913.6 \(\upmu\)g/g sample and 1377.0 \(\upmu\)g/g sample, the 241Am concentrations are 4548 \(\upmu\)g/g sample and 3155 \(\upmu\)g/g sample, and the 237Np concentrations are 300 \(\upmu\)g/g sample and 135.8 \(\upmu\)g/g sample in Metal-1 and Metal-2, respectively. All assay values are determined for a reference date of 1-March-2016.
Multiple plutonium chronometers and material production history
Model ages were determined using five chronometers, including four daughter–parent isotope pairs, 234U–238Pu, 235U–239Pu, 236U–240Pu and 241Am–241Pu, and the 237Np–241Pu granddaughter–parent pair. Daughter–parent model ages were calculated using the following simplified equation:
where t is the model age in years, \(\lambda_{\text{P}} \;{\text{and}}\;\lambda_{\text{D}}\) are, respectively, the decay constants of the parent and daughter nuclides in years−1, and R is the measured daughter/parent atom ratio on a reference date. The model date is calculated by subtracting the model age from the reference date. In using this model age equation, it is assumed that the material was completely free of daughter nuclides on the model date, so that the entire inventory of daughter nuclide measured in the sample was produced by radioactive decay of the parent isotope during time (t). It is also assumed that the material remained a closed system, with neither loss nor gain of parent nor daughter nuclide, since the time that the material was purified. If the material was incompletely purified of daughter nuclides, or if the material was contaminated with daughter nuclides since the time of purification, then the model age of the sample will be older than its purification age. The purification of a sample may precede or be coincident with the production of the material into the final form from which it is sampled for radiochronometry analysis.
The granddaughter–parent model ages are calculated using the full Bateman [12] decay equation. In contrast to the daughter–parent decay equation, the granddaughter–parent decay equation cannot be solved analytically for time (t). In practice, the model age is determined from the measured granddaughter/parent atom ratio by iteratively solving the Bateman equation for time (t), or through use of a ‘look-up’ table [e.g., 13]. As with the daughter–parent model age calculation, the granddaughter–parent model age is calculated under the assumption that that the material was purified of daughter and granddaughter nuclides on the model date, and that all daughter and granddaughter nuclides present in the material were produced by radioactive decay since that time. The following discussion will consider the case where daughter and granddaughter nuclides were present in the material at the time it was formed.
For Metal-1, the measured model dates range from 20-Jan-1959 ± 223 days (235U–239Pu) to 20-March-1960 ± 174 days (241Am–241Pu) (Fig. 1, Table 4). The model ages are concordant, with the exception of the 241Am–241Pu and 235U–239Pu model ages; the 235U–239Pu model age is resolvable from and older than the 241Am–241Pu model age at the calculated uncertainty limits, although the total difference at the uncertainty limits is only 15 days, which is a very small time interval relative to the model ages. The 238U–242Pu model age was also calculated, and this chronometer yields an impossibly old age of > 170,000 years, reflecting the presence of excess 238U in the sample. The initial abundance of 238U in the sample may be calculated using the following equation:
where t is the known age or assumed model age of the sample in years relative to reference date; Di is the atoms of daughter nuclide present in the sample t years before the reference date (initial daughter); Dm is the measured daughter atoms on the reference date; and Pi is the atoms of the parent nuclide present in the sample t years before the reference date, calculated by decay-correction of Pm, the measured atoms of parent nuclide on the reference date.
The concentration of initial 238U in Metal-1 is approximately 70 \(\upmu\)g 238U/g Pu, calculated using the 241Am–241Pu model date as the reference date; the 241Am–241Pu model date is used as the reference date because it is the youngest of the measured model dates. Two potential origins for this 238U excess are residual reactor fuel that was incompletely purified from the plutonium, and environmental uranium contamination. Environmental uranium is defined by a natural isotopic composition, and the addition of 70 ppm of natural uranium to this bulk plutonium material would have a negligible effect on the 234U and 236U contents and associated chronometers, and would result in a slight increase in the 235U–239Pu model age. The amount of initial 235U present in the sample (Di) may be estimated by assuming that the excess 238U in the sample has a natural isotopic composition, and a modified 235U–239Pu model age may be calculated using an expanded form of the model age equation that includes a term for initial daughter:
where Dm/Pm is the measured daughter–parent atom ratio on the reference date, and Di is calculated using Eq. (2). The model age of the sample, t, is determined through iteratively solving this expression. Although his approach yields a younger 235U–239Pu model age, it is still slightly older than the 241Am–241Pu model age, indicating that natural U contamination (either in the form of un-accounted for laboratory blank or contamination intrinsic to the Pu metal) can not be responsible for the minor offset between the 235U–239Pu and 241Am–241Pu model ages. Excess uranium with a 238U/235U ratio of approximately 2–3 will yield 235U–239Pu and 238U–242Pu model ages that are concordant with the 241Am–241Pu model age. The 241Am–241Pu chronometer yields the youngest model age, and represents the maximum (oldest) age of sample purification.
The consistency among the calculated model ages of Metal-1 provides a high degree of confidence in the interpretation that these represent the time at which the source Pu used to produce the Metal-1 sample was last completely purified of decay products. Taken together, this indicates that the method used to purify the bulk Pu effectively removed ingrown U, Am and Np. Aqueous processing methods, such as solvent extraction and ion exchange, were in common use for plutonium production in the late 1950s and early 1960s, and were likely used in the purification of this material [6,7,8]. Other purification methods, such as ER and MSE, were not in widespread use for plutonium production at this time [8]. Aqueous processing methods produce highly pure plutonium oxide, which was then commonly converted to plutonium metal using fluoride bomb reduction [18]. This conversion process does not result in further purification of actinides from the bulk plutonium, but rather can introduce impurities. Thus, the radiometric model ages determined for this sample likely represent the time of aqueous processing (e.g., PUREX method), and the small amount of natural uranium contamination in the sample may have been incorporated in the material during metal production.
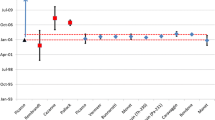
The model ages of Metal-2 are 15–20 years younger than Metal-1, and show a larger degree of variability than observed in Metal-1 (Fig. 2, Table 5). The model ages span nearly 7 years (exclusive of uncertainty limits), with model dates that range from 2-November-1974 ± 146 days (235U–239Pu) to 25-July-1981 ± 129 days (241Am–241Pu). The 238U–242Pu chronometer yields an impossibly old model age of > 40,000 years. As with Metal-1, the youngest model age is recorded by the 241Am–241Pu chronometer. This model age is approximately 6–7 years younger than the model ages determined with the 234U–238Pu, 235U–239Pu, and 236U–240Pu chronometers. The 237Np–241Pu chronometer yields an intermediate age, approximately 2 years older than the 241Am–241Pu model age. Taken together, these model ages indicate that Am was more completely purified than U and Np from the bulk Pu at some time between 25-July-1981 (the 241Am–241Pu model date) and 24-August-1982 (the date on which the sample was made).
Material production records indicate that Metal-2 was made on 24-August-1982. This date is more recent than any of the model dates determined for this sample, indicating that the sample contained initial 241Am, 237Np and U at this time. These initial values are calculated as the concentrations of decay products that are not supported by radioactive decay of Pu since 24-Aug-1982, using Eq. (2). This sample contained approximately 200 \(\upmu\)g/g initial 241Am, 26 \(\upmu\)g/g initial 237Np, and 280 \(\upmu\)g/g initial U relative to total Pu on 24-August-1982 (Fig. 3).
Model growth curves of 237Np/241Pu for Pu that contains no initial 237Np or 241Am (blue dot-dashed line), 200 ppm of initial 241Am (green dashed line), and 200 ppm initial 241Am and 26 ppm initial 237Np (solid red line). The model age calculated from the measured 237Np/241Pu of Metal-2, assuming that no initial daughter or granddaughter nuclides are present, is 36.85 years, which is older than known production age of 33.5 years (relative to reference date of 1-March-2016). When initial 241Am and 237Np are included in the decay model, the accurate Metal-2 model age of 33.5 years is calculated. (Color figure online)
The initial uranium in Metal-2 had a calculated isotopic composition of: 234U/235U = 0.0294 ± 0.0015 and 236U/235U = 0.221 ± 0.015, and an approximate 238U/235U of 0.12 (reference date for initial U is 24-August-1982). Initial uranium in the sample may potentially represent a mixture of uranium from a range of sources, including ingrown uranium from plutonium decay, residual uranium from reactor fuel, and natural uranium from environmental contamination. The calculated initial uranium isotopic composition is consistent with a mixture of plutonium from two sources: natural uranium and uranium produced from plutonium decay (Fig. 4). Specifically, the decay-produced uranium component is consistent with uranium that would be produced by decay of Metal-2 over the 7.5 years between the average U model date and the known sample production date. These calculated values are: 234U/235U = 0.03187 ± 0.00090 and 236U/235U = 0.2317 ± 0.0030 (k = 2). The isotopic composition of this uranium component indicates that the bulk plutonium was initially well-purified from the reactor fuel uranium, but that it contained a component of ingrown uranium at the time the sample was produced; that is, the bulk plutonium was not purified from uranium at the time of Pu metal production. The component of natural uranium contamination is constrained by the abundance of 238U, which is not significantly produced by Pu decay on the timescales involved in the nuclear era, in samples with low abundances of 242Pu. The uranium isotopic results were corrected for laboratory uranium blank, so this excess natural uranium component is in addition to laboratory blank. Although it is impossible to rule out a scenario in which the excess uranium components calculated for both Metal-1 and Metal-2 reflect a large degree of sample-to-sample variability in laboratory blank, these 238U excesses would require laboratory blank contributions to the samples that were 2–3 times greater than the blanks measured as part of this analytical campaign and during contemporaneous analyses performed in this laboratory. Thus, it is more likely that the natural uranium contamination resulted from a process that occurred prior to laboratory handling and analysis of the materials.
Isotopic compositions of U components in Metal-2. The isotopic composition of the initial U present in Metal-2 is calculated on the known sample production date of 24-August-1982. The isotopic composition of U produced from Pu decay during the 7.5 years between the average U model date and the known sample production date has the same 236U/235U, but slightly higher 234U/235U, and substantially lower 238U/235U than the calculated excess U. Error bars on the calculated ingrown U represent uncertainty associated only with the measured Pu isotopic composition (k = 2). The line represents mixing between the calculated ingrown U and natural U. The composition of initial U in Metal-2 is consistent with a mixture between ingrown and natural U components
The observation that the 241Am–241Pu model age is the youngest model age measured for Metal-2 indicates that the bulk Pu was purified within a year prior to metal production using a method that resulted in a higher decontamination factor for Am than for U or Np. The MSE purification method was developed in the 1960s to remove ingrown 241Am from older Pu, thereby reducing the gamma radiation associated with 241Am decay and increasing worker safety, and came into common use in the 1970s [7, 19, 20]. During the MSE process, Am is preferentially oxidized and extracted into a solvent molten salt, whereas U and Np are not oxidized and remain in the molten Pu metal [19]. In practice, MSE purification effectively separates Am from Pu, but does not significantly purify U or Np from Pu [J. McNeese, personal communication]. Purification by ER also came into common use in the 1970s, and is used to remove impurities other than Am from bulk Pu [9]. In the ER process, Pu is purified from U and Np, as well as Am. In general, the relative decontamination for these three actinides from the Pu metal product is greatest for Am, moderate for U, and least for Np [21; J. McNeese, personal communication]. Therefore, the relative model ages for Metal-2 calculated using the Am, Np and U decay products are inconsistent with purification by ER, but rather indicate that MSE was used to remove Am from the bulk Pu prior to casting.
Process knowledge of common plutonium metal production methods in use in the late-1970s and early 1980s indicate that Metal-2 likely has a complicated history. At that time, it was common practice to combine plutonium from batches with different storage and purification histories into a single batch during the metal casting process. Therefore, the model ages measured for Metal-2 represent an average history of the different plutonium batches that were mixed at the time of casting. Regardless, the relationships among the model ages provide signatures of the key processing methods that were used during the history of Metal-2. The observation that the three U–Pu model ages are significantly older than the 241Am–241Pu model age, and that the 237Np–241Pu model age is intermediate, indicates that ER was not a key process used to purify the plutonium prior to casting, but rather that the majority of the material was purified using MSE to remove Am shortly before casting. The three concordant uranium model ages indicate that all of the source plutonium was thoroughly purified from reactor fuel uranium, using aqueous processing methods (e.g., PUREX). The average date of this purification is 1975, though the purification dates of the individual batches of source plutonium may have spanned a larger range.
Conclusions
The use of multiple actinide radiochronometers for model age-dating Pu metal samples reveals details about the timing of and purification methods used during Pu metal production. In Metal-1, the model ages calculated using the 237Np–241Am–241Pu, 234U–238Pu and 236U–240Pu decay series yield concordant model ages, indicating that Np, Am and U were effectively removed from Pu around late-1959 to early-1960, most likely with an aqueous purification method. The small amount of initial natural U present in the bulk Pu, indicated by the calculated 238U–242Pu model age, was likely introduced during metal production. In Metal-2, the calculated model dates span a 6–7 year age range, from 1974 to 1981, all of which are older than the known sample production date in August 1982. Process knowledge of plutonium metal production at this time indicates that the Metal-2 sample likely represents a mixture of plutonium from different sources that were combined during the final stage of metal casting. Although the material is known to have a complex history, the model ages nonetheless reveal the key processes that were used to produce this sample. The three concordant U–Pu model ages indicate that the source plutonium was effectively purified from reactor fuel, with an average purification date of around 1975, and that this older plutonium component was purified of its ingrown Am, using MSE, in the early 1980s. These results for Metal-1 and Metal-2 indicate that the actinide series model-ages are potential signatures of the plutonium purification methods, and that these signatures may aid in identification of the origin of an unknown material in a nuclear forensic investigation.
References
Kristo MJ, Gaffney AM, Marks N, Knight K, Cassata WS, Hutcheon ID (2016) Nuclear forensic science: analysis of nuclear material out of regulatory control. Annu Rev Earth Planet Sci 44:555–579
Sturm M, Richter S, Agrebe Y, Wellum R, Mialle S, Mayer K, Prohaska T (2014) Evaluation of chronometers in plutonium age determination for nuclear forensics: what if the ‘Pu/U clocks’ do not match? J Radioanal Nucl Chem 302(1):399–411
Byerly BL, Stanley F, Spencer K, Colletti L, Garduno K, Kuhn K, Lujan E, Martinez A, Porterfield D, Rim J, Schappert M, Thomas M, Townsend L, Xu N, Tandon L (2016) Forensic investigation of plutonium metal: a case study of CRM 126. J Radioanal Nucl Chem 310:623–632
Rolison JM, Treinen KC, McHugh KC, Gaffney AM, Williams RW (2017) Application of the 226Ra-230Th-234U and 227Ac-231Pa-235U radiochronometers to uranium certified reference materials. J Radioanal Nucl Chem 314:2459–2467
Kayzar TM, Williams RW (2016) Developing 226Ra and 227Ac age-dating techniques for nuclear forensics to gain insight from concordant and non-concordant radiochronometers. J Radioanal Nucl Chem 307:2061–2068
Christensen EL, Gray LW, Navratil JD, Schulz WW (1983) Present status and future directions of plutonium process chemistry. In: Carnall WT, Choppin GR (eds) Plutonium chemistry. ACS Symposium Series 216. ACS Publications, Washington, DC, p 349
Nuclear materials, plutonium processing in the nuclear weapons complex (1992) United States General Accounting Office, GAO/RCED-92-109FS
Gray, LW(1999) From separations to reconstitution—a short history of plutonium in the US and Russia. UCRL-JC-133802
Mullins LN, Morgan AN (1981) A review of operating experience at the Los Alamos plutonium electrorefining facility, 1963–1977. LA-8943
Christensen DC, Mullins LJ (1983) Plutonium metal production and purification at Los Alamos. In: Carnall WT, Choppin GR (eds) Plutonium chemistry. ACS Symposium Series 216. ACS Publications, Washington, DC, p 409
Coops MS, Knighton JB, Mullins LJ (1983) Pyrochemical processing of plutonium. In: Carnall WT, Choppin GR (eds) Plutonium chemistry. ACS Symposium Series 216. ACS Publications, Washington, DC, p 381
Bateman H (1910) The solution of a system of differential equations occurring in the theory of radioactive transformations. Proc Camb Philos Soc 15(V):423–427
Moody KJ, Grant PM, Hutcheon ID (2015) Nuclear forensic analysis. CRC Press, Boca Raton, FL, p 502
Decay Data Evaluation Project. http://www.nucleide.org/DDEP.htm
National Nuclear Data Center, NuDat 2.6. http://www.nndc.bnl.gov/nudat2
Cheng H, Edwards RL, Shen C-C, Polyak VJ, Asmerom Y, Woodhead J, Hellstrom J, Wang Y, Kong X, Spötl C, Wang X, Alexander EC Jr (2013) Improvements in 230Th dating, 230Th and 234U half-life values, and U–Th isotopic measurements by multi-collector inductively coupled plasma mass spectrometry. Earth Planet Sci Lett. https://doi.org/10.1016/j.epsl.2013.04.006i
Jaffey AH, Flynn KF, Glendenin LE, Bentley WC, Essling AM (1971) Precision measurement of half-lives and specific activities of 235U and 238U. Phys Rev C 5:1889–1906
Moser WS, Navratil JD (1984) Review of major plutonium pyrochemical technology. J Less Common Metals 100:171–187
Knighton JB, Auge RG, Berry JW, Franchini RC (1976) Molten salt extraction of americium from molten plutonium metal. RFP-2365
Knighton JB, Steunenberg RK (1965) Distribution of transuranium elements between magnesium chloride and zinc–magnesium alloy. J Inorg Nucl Chem 21:1457–1462
Coops MS, Knighton JB, Mullins LJ (1982) Technology review report, pyrochemical processing of actinide metals. 184th National American Chemical Society Meeting, Kansas City, Missouri. UCRL-88116
Acknowledgements
This work was supported by the U.S. Department of Homeland Security and the U.S. Department of Energy. We thank Mike Blau and Jim McNeese for discussions on plutonium production methods. This work was performed under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344. LLNL-JRNL-748960.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gaffney, A.M., Wimpenny, J.B.N., Parsons-Davis, T. et al. A case study in plutonium radiochronometry using multiple isotope systems. J Radioanal Nucl Chem 318, 287–295 (2018). https://doi.org/10.1007/s10967-018-6131-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-018-6131-7