Abstract
The present study is to determine the activity concentration of radioactive nuclide in plant fertilizers used in agriculture in Iraqi Kurdistan region using high- purity germanium detector (HPGe) gamma spectrometer. The results showed that the range of activity concentrations for 226Ra, 232Th, 40K and 137Cs in chemicals and organic fertilizers are (0.1–134), (0.1–74), (1–12,000) and (0–1) Bq/kg respectively, based on the measured activities that were used to assess the radiological hazards. Radium equivalent activity in some samples exceeds the value (370 Bq/kg) which recommended by the OECD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In latest years, agricultural activities have extended widely, resulting in the applications of the different types of fertilizers. Chemical fertilizers are chemical compounds that provide necessary chemical elements and nutrients to the plants. Fertilizers have become important to the agricultural field all over the world [1]. It has been known that phosphate rocks contain high concentrations of uranium, thorium, radium and their decay products [2]. Phosphate rock is a main raw material used for manufacturing different types of phosphate fertilizers. Therefore, when this rock is processed into phosphates’ fertilizers, most of the radioactive nuclides come into the fertilizers. It has been estimated that phosphate fertilizers that applied to the fields could increase the radioactivity level in soils [3].
Fertilizers reorganize naturally occurring radioactive nuclides at trace levels through the environment and become a source of radiation. This phenomenon may result in potential radiological-hazard due to possible migration of elements of the plant fertilizers to soil and plants, and via the food chain, to human beings where this may lead to internal exposure through ingestion of food grown on fertilized soils [4].
The abnormal content of uranium, thorium and its decay products in ore rocks and fertilizer are the main sources of high radioactivity background parts that have been recognized in several areas of the world [5]. From the point of view of the international atomic energy agency of radioactive nuclides in food and the environment, it is necessary to measure the natural environmental radiation levels provided by ground, air, water, fertilizer etc. and to assess the dose limits of exposure to radiation from these naturally occurring radioactive nuclides [6, 7]. Human activities which involve the presence of natural radiation sources that lead to a significant increase in the exposure of workers, Regulation (EU) No 305/2011 requires information to be made available when products are placed on the market, and the current annual effective dose limits for occupational and public exposure should be maintained [8].
The aims of the present study are to determine the content of radioactivity in fifty different types of plant fertilizers that were commonly used in agriculture in Iraqi Kurdistan Region and to estimate their radiological risk to farmers and workers working in the a fertilizer factories and stores of the long-term exposure due to their application. Comparisons of the presented results were obtained with those of national and the world averages.
Experimental
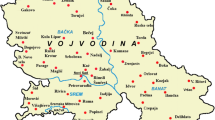
To determine the activity concentration of radioactive nuclides, fifty samples of plant fertilizers were collected from markets and companies in Iraqi Kurdistan Region. Properties of these samples were listed in Table 1. Kurdistan is located in the Middle East; it is situated in the north of Iraq, southeast of Turkey, northwest of Iran, and northeast of Syria, as shown in Fig. 1.
The samples were crushed and meshed perfectly to pass through 0.2 mm mesh. One kilogram from each sample was packed in a Marinelli beaker (one-liter size) for gamma spectrometry and sealed for 6 weeks to reach secular equilibrium between the radium contents of the sample and their daughter radionuclides [9, 10].
Each sample from fertilizers was subjected to a gamma-ray spectrometer with HPGe detector setup and the multichannel analyzer. The high- purity germanium detector is p-type of vertical closed-end coaxial, manufactured by PGT (Princeton Gamma Tec-PGT Company-USA) with the following specifications: Crystal diameter is 70.6 mm, crystal length 70.7 mm, resolution (FWHM) at 122 keV of 57Co is 1.18 keV and at 1332 keV of 60Co is 1.97 keV with relative efficiency 73.8% for this energy. The instrument calibrated for energy by using the standard point gamma-ray sources, such as 60Co (peaks 1173.2 and 1332.5 keV), 137Cs (peak 661.7 keV) and 226Ra (peaks 186.1, 295, 351.9, 609, 665, 1120 and 1764 keV). The efficiency calibration was achieved using the same three standard sources, and the relative efficiency curve was normalized to absolute volume efficiency curve, using two radioactive samples prepared [potassium chloride KCl powder and of Uranylace (UO2(OCOCH3)2·2H2O) solution] to satisfy the geometrical conditions of the tested samples.
The detector was placed in a wall of lead with thickness (10 cm) to shield the measuring station against background radioactivity. The samples were placed over the detector for at least 10 h. The spectra were evaluated by Thermo Scientific System 8000 multi-channel analyzer and the computer software program (Quantum Gold 2001 for PGT Company). In order to determine the background distribution in the environment around the detector, an empty sealed beaker was counted in the same manner and in the same geometry as the samples. The measurement time of activity or background was 10 h. (The background spectra were used to correct the net peak area of gamma rays of measured isotopes). After measurement and subtraction of the background, the activity concentration was calculated from following gamma-ray photopeak lines [11, 12].
-
(a)
226Ra activity concentration was calculated as the weighted average of the activity determined, using the gamma-ray lines 351.9 (35.8%) keV gamma-rays from 214Pb decay, 609.3 (44.8%), 1120 (14.8%) and 1764.5 keV (15.36%) gamma-rays from 214Bi decay.
-
(b)
The gamma-ray photo peaks used for the determination the activity concentration of the 232Th were 238.6 keV (43%) from 212Pb decay, 583 keV (84.5%) and 2614.5 keV (99.16%) from 208Tl decay and 911.2 keV (26.6%) from 228Ac decay.
-
(c)
The activity concentration of 40K was directly determined using 1460.8 (10.7%) gamma-rays line.
-
(d)
The activity concentration of 137Cs was directly determined using 661.7 (85.21%) gamma-rays line.
Theory
Activity concentration
Activity concentration of the isotopes calculated using the following formula [13, 14]:
where ε is the absolute gamma peak efficiency of the detector to its particular gamma-ray energy, Iγ is the decay intensity of the specific energy peak (including the decay branching ratio information), T is the counting time of the measurement in second, M is the mass of the sample in kg and SD is the standard deviation of the net count rate per second.
Radium equivalent activity (Raeq)
The total exposure to radiation from these naturally occurring radioactive nuclides was defined in terms of radium equivalent activity (Raeq) in (Bq/kg) and used to assess the gamma-ray radiation hazards due to specified radionuclides 226Ra, 232Th and 40K. The Raeq of a sample in (Bq/kg) can be achieved using the following relation [15, 16]:
where ARa, ATh, AK are the activity concentrations of 226Ra, 232Th and 40K in a unit (Bq/kg) respectively. The published maximal permissible Raeq is 370 Bq/kg [17, 18].
The absorbed gamma dose rates (DR)
The absorbed gamma dose rates in air at 1 m above the ground surface for the uniform distribution of naturally occurring radioactive nuclides were calculated based on guidelines provided by [19]:
Annual effective dose (AED)
In order to estimate the annual effective dose rate in the air, the conversion coefficient from the absorbed dose in the air to the effective dose received by an adult must be considered. Annual estimated average effective dose (AED) received by an individual was calculated using a conversion factor of 0.7 Sv/Gy, which was used to convert the absorbed rate to the human effective dose. The annual effective dose is determined using the following equations [15, 16]:
where DR is the calculated dose rate (in nGy/h), T is the occupancy time and F is the conversion factor, this value is published in UNSCEAR 1993 and UNSCEAR 2000 [20, 21], to be 0.7 Sv/Gy for environmental exposure to gamma rays of moderate energy. The outdoor occupancy factor T is about (20% of 8760 h/y). The outdoor annual effective dose is given by the following equation:
The world average annual effective dose (AED) from outdoor or indoor terrestrial gamma radiation only is 70 μSv/y [21].
External hazard index (Hex)
The external hazard index is an evaluation of the hazard caused by gamma-rays emitted from natural radioactive nuclides. It was calculated by assuming that 370 Bq/kg of 226Ra or 259 Bq/kg of 232Th or 4810 Bq/kg of 40K produces the same gamma-ray dose rate. The prime objective of this index is to limit the radiation dose to the admissible permissible dose equivalent limit around 1 mSv/y. In order to evaluate this index, one can use the following relation [15, 18]:
This model takes into consideration the maximum value of external hazard which is caused by gamma-rays corresponds to a radium equivalent activity of 370 Bq/kg [17, 18].
Excess lifetime cancer risk (ELCR)
Excess lifetime cancer risk measures the additional cancer risk induced by exposure to ionizing radiations. Based on the calculated values of AED, ELCR is calculated using the equation [22, 23]:
where AED is the annual effective dose, DL is the average duration of life which is 70 year, RF is the risk factor given as 0.05 by ICRP 1991 [24].
Results and discussion
The activity concentrations of 226Ra, 232Th, 40K, and 137Cs in different fertilizer samples were measured and listed in Table 2. The results showed that the mean value and a range of activity concentrations of 226Ra, 232Th, 40K and 137Cs in these fertilizers are 7 ± 1 (0.1–134), 4 ± 1 (0.1–74), 2500 ± 100 (1–12,000) and 0.1 ± 0.01 Bq/kg (0–1) Bq/kg respectively. The highest value of activity concentrations of 226Ra and 232Th are found in NPK fertilizer, while the lowest values were found in urea and calcium fertilizers. Except for fertilizer samples (NPK1, NPK2, NPK3, OR3, and OR4), the measured value of activity concentrations of 226Ra and 232Th are lower than the word wide average value of 35 and 30 Bq/kg for 226Ra and 232Th respectivily [21]. The variation of radionuclides activities in studying fertilizers may be due to the different origins of raw material and the chemical processes during manufacturing of the fertilizer.
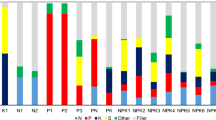
As shown in Fig. 2, the activity concentration of 226Ra in most studied fertilizer samples are higher than the activity concentration of 232Th in these samples, which may be due to the accumulation of dissolved uranium and its products, in the form of urinal complex in the seawater during geological formation of the phosphate rocks [25].
Figures 3 and 4 shows the relationships between activity concentrations of 226Ra and 232Th with concentration of P2O5 in fertilizer samples. It is clear that there is no linear relationship between activity concentrations of 226Ra and 232Th and concentration of P2O5 in fertilizer samples, and the variation of radionuclides activities in studying fertilizers due to different origins of raw material and the chemical processes during the manufacturing of the fertilizer.
The highest value of activity concentration 40K was found in potassium sulfate fertilizer, while the lowest value was found in urea and calcium fertilizers. This is due to high concentration of K2O in potassium sulfate fertilizer, because there is a good relationship between concentration of K2O and 40K radioactive isotope content, and the presence of radioactive 40K, whose natural abundance in natural potassium K is 0.0118%, and this relation was found in Fig. 5. The activity concentration of 40K in potassium sulfate and NPKs fertilizer samples was higher than the word wide average value for 40K which is 400 Bq/kg [21]. While the activity concentration of 40K in Urea, Calcium, NP, and organic samples, was lower than the word wide average value. The activity concentration of 137Cs was found only in organic fertilizer samples, this may be due to the production process of the organic fertilizers. The results for the activity concentration of 226Ra, 232Th, 40K, and 137Cs, of the present work, compared with other studies are presented in Table 3.
In order to compare the activity concentrations of 226Ra, 232Th and 40K in fertilizer samples, the radium equivalent activity (Raeq) as a common index was used to obtain the sum of activities. To estimate the radiological risk to farmers and workers working in a fertilizer factory of the long-term exposure due to their application, the external absorbed dose rate (D), annual effective dose (AED), external hazard index (Hex) and excess lifetime cancer risk (ELCR) for different types of fertilizer samples were calculated and listed in Table 4.
The value of radium equivalent activity (Raeq) in the fertilizer samples varied from (0.4 to 900) Bq/kg, the variation of radium equivalent activity (Raeq) in studying fertilizers, due to the different amount content of 226Ra, 232Th and 40K in fertilizers. The calculated value of radium equivalent (Raeq), except for fertilizer samples (K1, NPK1, NPK5, NPK6, NPK7 NPK12, NPK13, NPK15, NPK18 NPK20, NPK24 and L1), were lower than the recommended level of 370 Bq/kg [17, 18, 31].
The calculated value of the external absorbed dose rate (DR) of the fertilizer samples varied from (0.2 to 480) nGy/h. The value of absorbed dose rate (DR) in Potassium Sulfate, Potassium Nitrate and NPK complex fertilizers were higher than the worldwide average value, while the value of absorbed dose rate (DR) in urea, diammonium phosphate, urea ammonium phosphate, calcium nitrate and organic were lower than the worldwide average value of 59 nGy/h [21].
The calculated values of outdoor annual effective dose (AED) in the fertilizer samples varied from (0.2 to 590) µSv/y. The calculated value of annual effective dose in potassium sulfate, potassium nitrate and NPK complex fertilizers were higher than the worldwide average value, while the measured value of in urea, diammonium phosphate, urea ammonium phosphate, calcium nitrate and organic were lower than the worldwide average value of 70 μSv/y [21].
The calculated value of external hazard index (Hex) in the fertilizer samples varied from (0 to 2.4) mSv/y, the data indicated that the value of external hazard index in fertilizer samples (K1, NPK1, NPK5, NPK6, NPK12, NPK13, NPK18, NPK24, L1) were higher than the safety limits (1 mSv/y) [14, 15, 17]. While the value of external hazard index in other fertilizer samples within the safety limit. Thus, the use of chemical fertilizers (K1, NPK1, NPK5, NPK6, NPK12, NPK13, NPK18, NPK24, L1) in large extent can be harmful to the health of farmers and workers working in a fertilizer factory.
The calculated value of excess lifetime cancer risk (ELCR) in the fertilizer samples varied from (0 to 20 × 10−4)/person. The results of the present study indicated that the use of plant fertilizers to enhance crop yield enhances the concentration of natural radioactive nuclides in soil and hence the exposure of farmers working in the fields.
No linear relationships have been found between external hazard index (Hex) with 226Ra and 232Th activity concentrations, but present result in Fig. 6 shows a good correlation between external hazard index (Hex) and 40K activity concentration with a correlation coefficient of (R2 = 0.98), this means that most of the hazard caused by nuclear radiation from fertilizer samples were coming from 40K radioactive nuclide (or by the high concentration of 40K radioactive nuclei in the fertilizer samples).
Conclusions
The activity concentration of radioactive nuclide and related radiation hazards in plant fertilizer samples being used in Iraqi Kurdistan Region were determined by using a HPGe gamma spectrometer.
Except for fertilizer samples (NPK1, NPK2, NPK3, OR3, and OR4), the measured value of activity concentrations of 226Ra and 232Th are lower than the word wide average value. The activity concentration of 40K in potassium sulphate and NPKs fertilizer samples was higher than the world average value, while its activity in urea, calcium, NP and organic samples was lower than the world average value. No relations have been found between activity concentrations of 226Ra and 232Th with the concentration of P2O5 in fertilizer samples. The use of potassium sulphate and NPKs fertilizer made the calculated value of external hazard index (Hex) higher than the recommended level and harmful for the health of farmers and workers working in a fertilizer factories and stores. On the other hand, fertilizer samples of urea, calcium nitrate, urea ammonium phosphate, di-ammonium phosphate and organic were safer for agriculture and they have a low effect on human health.
References
Uosif MAM, Mostafa AMA, Elsaman R, Moustafa E (2014) Natural radioactivity levels and radiological hazards indices of chemical fertilizers commonly used in Upper Egypt. J Radiat Res Appl Sci 7:430–437. https://doi.org/10.1016/j.jrras.2014.07.006
Skorovarov JI, Rusin LI, Lomonosov AV et al (1996) Development of uranium extraction technology from phosphoric acid solutions with extractant PN-1200. Int At Energy Agency Vienna I:106–113
Hussein A (1994) Determination of uranium and thorium concentration in some Egyptian rock samples. J Radioanal Nucl Chem 188:255–265
Shafi-ur-Rehman Nadia I, Munazza F et al (2006) Determination of 238U contents in ore samples using CR-39-based radon dosimeter—disequilibrium case. Radiat Meas 41:471–476. https://doi.org/10.1016/j.radmeas.2005.10.002
Al-Jundi J, Al-Bataina BA, Abu-Rukah Y, Shehadeh H (2003) Natural radioactivity concentrations in soil samples along the Amman Aqaba Highway, Jordan. Radiat Meas 36:555–560. https://doi.org/10.1016/S1350-4487(03)00202-6
Jebur JH, Subber ARH (2014) Natural radioactivity of some local and imported fertilizer in Basrah Governorate/Iraq. Arch Phys Res 5:18–22. http://scholarsresearchlibrary.com/archive.html
IAEA (1989) Measurement of radionuclides in food and the environment. A guidebook. International Atomic Energy Agency IAEA, Vienna
European Commission (2014) Council Directive 2013/59/Euratom of 5 December 2013 laying down basic safety standards for protection against the dangers arising from exposure to ionising radiation, and repealing Directives 89/618/Euratom, 90/641/Euratom, 96/29/Euratom, 97/43/Euratom a. Off J Eur Commun L13:1–73. https://doi.org/10.3000/19770677.L_2013.124.eng
Hamby DM, Tynybekov AK (2002) Uranium, thorium, and potassium in soils along the shore of Lake Issyk-Kyol in the Kyrghyz Republic. Environ Monit Assess 73:101–108. https://doi.org/10.1097/00004032-199910000-00010
Hussain RO, Hussain HH (2011) Investigation the natural radioactivity in local and imported chemical fertilizers. Brazilian Arch Biol Technol 54:777–782. https://doi.org/10.1590/S1516-89132011000400018
Raheem EH (2015) Activity concentration of natural radioactivity and dose assessment for brands of chemical fertilizers used in Iraq. Int J Curr Eng Technol 5:3823–3828
Al Mugren KS, El-Taher A (2016) Risk assessment of some radioactive and elemental content from cement and phosphate fertilizer consumer in Saudi Arabia. J Environ Sci Technol 9:323–328. https://doi.org/10.3923/jest.2016.323.328
Gilmore GR (2011) Practical gamma-ray spectrometry, 2nd edn. Wiley, Warrington, DC
Samad MA, Ali MI, Paul D, Islam SMA (2012) Assessment of radioactivity in the wastes generated from the diammonium phosphate (DAP) fertilizer factory, Chittagong, Bangladesh. Jahangirnagar Univ Environ Bull 1:15–24
Alzubaidi G, Hamid FBS, Abdul Rahman I (2016) Assessment of natural radioactivity levels and radiation hazards in agricultural and virgin soil in the state of Kedah, north of Malaysia. Sci World J. https://doi.org/10.1155/2016/6178103
Hassan NM, Chang BU, Tokonami S (2017) Comparison of natural radioactivity of commonly used fertilizer materials in Egypt and Japan. J Chem. https://doi.org/10.1155/2017/9182768
OECD (1979) Exposure to radiation from the natural radioactivity in building materials. Report by an NEA group of experts, Paris
Hameed PS, Pillai GS, Mathiyarasu R (2014) A study on the impact of phosphate fertilizers on the radioactivity profile of cultivated soils in Srirangam (Tamil Nadu, India). J Radiat Res Appl Sci 7:463–471. https://doi.org/10.1016/j.jrras.2014.08.011
EC-European Commission (1999) Radiological protection principles concerning the natural radioactivity of building materials. Radiat Prot 112:1–16
UNSCEAR (1993) Sources and effects of ionising radiation. United Nations Scientific Committee on the Effects of Atomic Radiation, Report to the General Assembly, with Scientific Annexes. United Nations, New York
UNSCEAR (2000) Sources and Effects of Ionizing Radiation, United Nations Scientific Committee on the Effects of Atomic Radiation UNSCEAR 2000 Report to the General Assembly, with Scientific Annexes. United Nations, New York
Qureshi AA, Tariq S, Din KU et al (2014) Evaluation of excessive lifetime cancer risk due to natural radioactivity in the rivers sediments of Northern Pakistan. J Radiat Res Appl Sci 7:438–447. https://doi.org/10.1016/j.jrras.2014.07.008
El-Bahi SM, Sroor A, Mohamed GY, El-Gendy NS (2017) Radiological impact of natural radioactivity in Egyptian phosphate rocks, phosphogypsum and phosphate fertilizers. Appl Radiat Isot 123:121–127. https://doi.org/10.1016/j.apradiso.2017.02.031
International Commission on Radiological Protection (1991) ICRP Publication 60: 1990 Recommendations of the International Commission on Radiological Protection
Khater AEM, AL-Sewaidan HA (2008) Radiation exposure due to agricultural uses of phosphate fertilizers. Radiat Meas 43:1402–1407. https://doi.org/10.1016/j.radmeas.2008.04.084
Boukhenfouf W, Boucenna A (2011) The radioactivity measurements in soils and fertilizers using gamma spectrometry technique. J Environ Radioact 102:336–339. https://doi.org/10.1016/j.jenvrad.2011.01.006
Jibiri NN, Fasae KP (2011) Activity concentrations of 226Ra, 232Th and 40K in brands of fertilisers used in Nigeria. Radiat Prot Dosimetry 148:132–137
Alharbi WR (2013) Natural radioactivity and dose assessment for brands of chemical and organic fertilizers used in Saudi Arabia. J Mod Phys 2013:344–348. https://doi.org/10.4236/jmp.2013.43047
Elisha JJ, Yisa J, Adeyemo DJ (2013) Radiological analysis of selected organic fertilizers in Zaria local government area council, Kaduna State, Nigeria: possible health implications\n. IOSR J Appl Phys 5:44–48
Hussain HH, Farhan AH (2011) Estimation of radiation levels due to organic agricultural fertilizers. J Coll Educ Univ Kufa 3:509–521
Alaamer AS (2008) Assessment of human exposures to natural sources of radiation in soil of riyadh, Saudi Arabia. Turkish J Eng Environ Sci 32:229–234. https://doi.org/10.1093/oxfordjournals.rpd.a032637
Acknowledgements
The authors acknowledge the Directorate of Agriculture Research-Erbil- Ministry of Agriculture, the center of scientific research Salahaddin University-Erbil, and the center of Nuclear Research Koya University for their help in conducting this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Azeez, H.H., Ahmad, S.T. & Mansour, H.H. Assessment of radioactivity levels and radiological-hazard indices in plant fertilizers used in Iraqi Kurdistan Region. J Radioanal Nucl Chem 317, 1273–1283 (2018). https://doi.org/10.1007/s10967-018-6001-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-018-6001-3