Abstract
A phosphate groups bearing thin polymer film has been anchored on Teflon by radiation induced grafting and subsequent chemical modification. Thus formed phosphate-g-Teflon (Ph-g-T) sheet has been characterized appropriately and studied for its selectivity towards Pu(IV) and U(VI) ions at different acidities. Depending upon acidity dependent selectivity of Ph-g-T toward actinides ions, the solid state nuclear track detector based methods was developed to quantify Pu(IV) and U(VI) at ultra-trace concentration in a variety of aqueous samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The quantifications of alpha emitting and fissile elements in the aqueous samples such as environmental samples, radiopharmaceutical samples derived from fission molly, fuel reprocessing aqueous waste samples and nuclear forensic samples often require highly sensitive radiation measurement instrumentation hyphenated with appropriate sample purification and source preparation methods to avoid possible interferences [1,2,3,4,5]. However, in many such applications, the sample is to be subjected to a preconcentration step for bringing the concentration of analyte in the measurement range of the radioanalytical method [1,2,3,4,5]. For example, the dangerous level for accumulated Pu in the human body is 10–12 g g−1 (1000 fg g−1). This highlights the compulsion to monitor much lower levels in the surrounding environment in order to evaluate the possibility of bio-accumulation of radiotoxic elements [6].
To combine multiple sample manipulation steps such as purification, preconcentration and source preparation, the polymer sorbent based thermal ionization mass spectrometry has been developed for quantifying uranium(VI) and plutonium(IV) ions in a variety of aqueous samples [7]. Similarly, thin sorbent films anchored on the silicon, polymer, membrane, glass have been developed using different approaches for one step sample manipulation and subjecting thin film sorbents directly to alpha spectrometry [8,9,10,11,12,13,14,15]. To process large volume of aqueous samples, a surface grafted poly(ether sulphone) membrane has been developed for ultrafiltration to capture the alpha emitting actinides and subsequently quantifying by subjecting the membrane to alpha spectrometry [16]. The scintillating polymer membranes have also been developed that produce scintillation proportion to the alpha particles emitted by the actinides captured in the membrane matrix [17, 18].
Solid state nuclear track detectors (SSNTDs) are the most sensitive for recording energetic charge particles but lack chemical selectivity and have limited capability to distinguish the particles based on their energies unlike alpha spectrometry. However, a combination of alpha spectrometry and fission track analysis could be very effective for nuclear forensic applications [19]. SSNTDs have been used for monitoring alpha particles from radon decay [20], detecting residual hot particles after nuclear facility decommission activities [21], and determination of alpha emitting radionuclides in the marine fish samples to study the pathways of 238U, 232Th, 222Rn, and 220Rn to human body [22]. To improve analytical applications of SSNTDs, a thin polymer inclusion membrane supported on the silanized glass and surface phosphate functionalized polymer grafted membranes have been used for pre-concentrating actinides selectively and used as a source for registering the alpha tracks in CR-39 detector [23, 24]. In general, the phosphate groups are selective to the tetravalent and hexavalent actinides ions at higher HNO3 concentration but lack the selectivity towards Pu(IV) ions. It has been reported that the selectivity of the phosphate groups for covalent binding with Pu(IV) could be influenced by the functional groups in a close proximity [25, 26]. In these work, it has been shown that the high selective toward Pu(IV) ions could be achieved by choosing appropriately the chemical architecture of the functional polymer film.
In the present work, the gylycidyl methacrylate (GMA) has been anchored on the Teflon film by mutual radiation grafting technique reported elsewhere [27, 28]. The grafting extent of the poly(GMA) on Teflon has been controlled by the exposure to appropriate absorbed γ-dose from 60Co and other experimental parameters. The epoxide ring of the grafted GMA was later converted to phosphoric acid under optimized chemical conditions. Thus synthesized Ph-g-T has been characterized and used for the selective pre-concentration of Pu(IV) and U(VI) in the aqueous samples and subsequent direct determinations by registering alpha and fission tracks in CR-39 and Lexan detector. The schematic representation of the efficacy of the Ph-g-T in combining sample treatment and source preparation for the radiation measurements based analytical methods is illustrated in the Scheme 1.
Experimental
Reagents and apparatus
Teflon sheet (Thickness = 0.5 mm) used in the present work was procured from M/s Hindustan Flurocarbons, Hyderabad, India. Glycidyl methacrylate monomer (purity > 97%) was obtained from M/s Otto Chemie Private Limited, Mumbai. All other chemicals used were of analytical reagent grade (Purity > 99%), and obtained from S.D. Fine Chem. Limited, India. Gamma irradiator GC-5000 having 60Co γ-source, supplied by Board of Radiation and Isotope Technology, Mumbai, India was used for delivering desired dose for grafting with suitable lead attenuators. The dose rate of gamma chamber was determined to be 1 kGy h−1 by Fricke dosimetry prior to grafting experiments. The SSNTDs used were CR-39 (allyl diglycol polycarbonate) (500 µm thickness) and Lexan (bisphenol-A polycarbonate) (200 µm thickness) plastics sheets obtained from Global Nanotech, Mumbai, India and General Electric Co., USA, respectively. The developed alpha and fission tracks were observed under Olympus fully motorized transmission optical microscope (model no. BX63, Olympus Tokyo, Japan) attached with QIMAGING QICAM CCD camera and cellSens Dimension Package for image analysis. The purified stock solution of research reactor grade Pu was from our lab Radiochemistry Division, BARC, Mumbai, India. The isotopic composition of Pu (at.%) was as 238Pu (0.16 ± 0.006), 239Pu (68.79 ± 0.03), 240Pu (26.94 ± 0.03), 241Pu (2.09 ± 0.005), and 242Pu (2.02 ± 0.006). The α-activity was measured with a home-built liquid scintillation counter, and alpha spectrometer equipped with a passivated ion-implanted planar silicon (PIPS) detector (Canberra, PD-450-16-100AM) with an area of 450 mm2 and a resolution of 16 keV (FWHM) at 5.486 MeV of 241Am connected to a multi-channel analyzer. The scintillation cocktail W (Sisco Research Laboratories Pvt. Limited, Mumbai) was used for the scintillation counting. FTIR spectra were recording using JASCO 420 spectrometer, and STARe system METLER TOLEDO instrument (Model 92-16.18) was used for obtaining thermograms at heating rate 5 K min−1 under flowing argon atmosphere. Surface topographies of grafted samples were studied using a Nanotec Cervantes FullMode AFM. A Si probe on a rectangular cantilever was used to obtain images in a dynamic mode (amplitude modulation).
One side functionalization of Teflon sheet
Teflon sheets were washed first with toluene followed by detergent solution, and finally vacuum dried at 50 °C and stored in desiccator for further use. The cleaned Teflon sheet had a bulk density = 2.1 g/cc, and surface energy = 22.5 mJ m−2. In order to avoid contact of one face of Teflon with grafting solution so that grafting occurs on only one face of Teflon, Teflon pieces (1.5 × 2.0 cm2) of Teflon were compression molded into 0.7 mm poly(propylene) sheet at 180 °C and 98,066.5 Pa pressure. The samples immersed in the GMA containing solution were then irradiated in the gamma chamber for the required doses. Thus obtained GMA-g-Teflon was refluxed for 3 h at 80 °C in a solution of phosphoric acid and tetrahydrofuran (75:25 v/v). The Ph-g-T samples were Soxhlet extracted with acetone for 8 h to remove any trapped homopolymer of GMA in the grafted Ph-g-T. Finally, the grafted matrix was washed with water and dried in vacuum at 50 °C for further use.
Representative actinide ions sorption studies
The sorption efficiencies of Ph-g-T pieces (1 × 1 cm2) toward representative actinide ions were carried out using 241Am(III), 233U(VI) and mixPu(IV) in 5 mL of solution having varying concentrations of HNO3. The extents of Pu(IV), U(VI) and Am(III) sorptions on Ph-g-T were quantified using liquid scintillation counting of aqueous samples (100 µL) taken from the solutions before and after overnight equilibration and added in 5 mL scintillation cocktail-W. The sorption efficiency (uptake) of actinide was obtained from Eq. (1):
where Ab and Af were alpha scintillation count rates of mixPu, 233U and 241Am in the solution before and after equilibration with Ph-g-T, respectively. The sorption experiments were also carried out by stirring the solution for a fixed period of time, and the percentage uptakes of mixPu, 233U and 241Am radiotracers as the function of time were monitored.
SSNTD experiments
Alpha tracks were registered from the Pu(IV)-loaded Ph-g-T sheet (1 × 1 cm2) in CR-39 using 2π contact geometry. After exposure to the Pu(IV)-loaded Ph-g-T for a fixed time period, CR-39 detectors were chemically etched with 6 mol L−1 NaOH at 70 °C for 5 h to reveal alpha tracks for the observations under optical microscope. Similarly, the fission tracks were recorded by irradiating Pu(IV)-loaded Ph-g-T sheet (1 × 1 cm2) in Lexan with a 2π contact geometry in neutron irradiation facility at BARC having neutron flux ≈ 107 cm−2 s−1. The fission tracks in Lexan were developed by chemical etching with 6 mol L−1 NaOH at 60 °C for 1 h.
Results and discussion
Formation of Ph-g-T
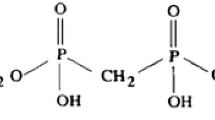
The gamma rays induced mutual grafting of poly(GMA) on one surface of Teflon sheet was carried out by blocking one of the Teflon surfaces. The details regarding the grafting extent, absorbed dose, dose rate effect, rate of grafting etc. had been provided in our earlier reported work [28]. The grafted poly(GMA) was reacted with phosphoric acid at elevated temperature to convert epoxide ring of the grafted GMA to generate phosphate groups (Scheme 2). The chemistry of chemical conversion of epoxide to different functional group is well established and reported. Ester bond of epoxide ring can be hydrolyzed or converted to phosphoric group depending on acid used as well as other experimental conditions. In the present work, the method reported by Kalal et al. [29] was used to attach phosphate groups by epoxy ring opening reaction. It is clear from chemical structure of thus formed Ph-g-T that hydroxyl groups are in a close proximity and may lead to hydrogen bonding. The hydrogen bonding is known to influence the affinity of phosphate and diglycolamide groups toward f-element ions [30, 31].
Characterizations of Ph-g-T
Fourier transform infrared analysis
The presence of -H2PO4 in Ph-g-T used for adsorption studies was confirmed by comparing FTIR spectra of pristine Teflon, GMA-g-Teflon and Ph-g-T given in Fig. 1. In FTIR spectrum of pristine Teflon, the absorbance at 1145 and 1205 cm−1 were assigned to F2 stretching. Grafting of GMA was confirmed by the presence of absorption band at 1737 cm−1 assigned to the C–O stretching of the ester group and symmetrical stretching of the epoxy ring near 1250 cm−1. Another band in the region 950–800 cm−1 was attributed to asymmetrical ring stretching in which C–C bond was stretched during contraction of the C–O bond. The C–H stretching vibrations of epoxy rings could be observed in 3050–2950 cm−1 region. The conversion of epoxy to phosphate group was established by appearance of band in the region 900–1040 cm−1 which was assigned to P–OH stretching vibration and was further confirmed by -OH group functionality from peaks at 3200 and 3700 cm−1.
Thermogravimetric analysis
The thermograms of pristine Teflon showed sharp weight loss near its decomposition temperature 480 °C which is similar to that reported elsewhere [28]. The thermogram of GMA-g-Teflon showed two step weight losses at temperatures 230 and at 480 °C. The additional weight loss step in grafted Teflon was attributed to grafted poly(GMA) chains [28, 32]. The phosphate converted grafted Teflon showed multisteps gradual weight losses. The quantitative analyses of weight losses observed due to grafted poly (GMA) and phosphate group were found to be 2.23 wt% and approximately 0.5 wt% which were in a good agreement with extent of weight gain measured using gravimetric measurement (Fig. 2).
AFM analysis
The change in surface morphologies of grafted Teflon substrates were studied by AFM analysis. It is evident from the representative AFM images given in Fig. 3 that the surface became rough after grafting and chemical modification. This was expected due to formation of thin poly (GMA) layer on Teflon surface due to grafting.
Pu and U sorption studies
The acidic phosphates are known to have affinity toward actinide ions [25, 26]. Therefore, the Ph-g-T sheets were equilibrated in the solutions having UO22+ ions at pH = 2 and Pu4+ ions at 3 mol L−1 HNO3. The Pu4+-loaded Ph-g-T sheet was subjected to alpha radiography using CR-39 to study the distributions of Pu4+ ions bound with phosphate groups on the grafted matrix. Representative image obtained by transmission optical microscope is shown in Fig. 4a. It was observed that alpha tracks were uniformly distributed (± 8%) indicating homogeneity of grafting, thus a small representative area could be counted for obtaining alpha track density. To study distribution of U(VI) ions, the UO22+-loaded Ph-g-T sheet was kept in a 2π contact with Lexan and irradiated with reactor neutron to register the fission tracks. It was seen from developed fission tracks shown in Fig. 4b that U(VI) ions were also distributed uniformly on the grafted surface of Ph-g-T sheet.
To optimize the maximum extent of surface grafting, the Ph-g-T substrates with different phosphate contents (0.5–2.1%) were prepared by controlled grafting extent of GMA using the experimental parameters described elsewhere [28]. These Ph-g-T substrates were loaded with Pu4+ ions from 3 mol L−1 HNO3 and subjected to alpha spectrometry. From alpha spectrum of Pu4+ loaded Ph-g-T (Fig. 5) it was observed that, the characteristics alpha energy peaks corresponding to 239 Pu, 240Pu and 238Pu were well resolved with left-side tailing. This is an indicative of smaller extent of loss of kinetic energy of alpha particles in the grafted polymer matrix itself before emerging out. It was also observed that the extent of energy degradation of alpha particles increased with increase in the % phosphate content (i.e. polymer grafting). This could be attributed to increase in the thickness of phosphate bearing grafted layer with increase in grafting extent. The full width at half maxima (FWHM) from the alpha spectrum recorded was taken as indicative of the extent to which the alpha particles lose its energy while coming out from the matrix. It was observed that that FWHM value of 0.5 and 1% phosphate bearing Ph-g-T was similar (0.22 MeV). In case of 1.2% phosphate, FWHM increased to 0.26 MeV. FWHM was further increased in 2.1% phosphate content of the sample (0.40 MeV). This suggested significant deterioration of energies of alpha particles in the matrix having > 1% phosphate bearing polymer layer on Ph-g-T. As clear from Fig. 5, there was significant contribution of alpha counts below 4 MeV in case of the 1, 1.2, 2.1% phosphate containing Ph-g-T samples due to left side tailing. Therefore, further studies were done using 0.5% phosphate bearing Ph-g-T to avoid registering alpha particles in solid state nuclear track detector (SSNTD) having varying energies. As such, the track density is not affected by loss of small amount of kinetic energy of alpha particles in the source matrix. The alpha track density is used for quantification using SSNTD. In alpha spectrometry, the quantification would be affected by left side tailing due to erroneous integration of a peak area.
The acidic phosphate groups are expected to bind with actinide ions via ion-exchange complexation with P–O– at pH range (1–7.5) and complexation of neutral nitrate actinide salt with P=O at higher HNO3 concentration. Thus, it is expected that Ph-g-T would exhibit a HNO3 concentration dependent selectivity toward actinide ions. It was seen from the uptake profiles of the representative ions such as Am3+, UO22+ and Pu4+ ions (Fig. 6) that Pu4+ ions sorb preferentially above 3 mol L−1 HNO3. This could be attributed to ability of Pu4+ ions to coordinate with nitrate ions [26] and form neutral complex with P=O group. The trivalent actinide ions such as Am3+ ions bind with phosphate groups via ion-exchange mechanism, and, therefore, sorbs at pH range (1–7.5). However, it is interesting to note that UO22+ ions did not sorb at higher HNO3 concentration.
The acidic phosphate groups are expected to bind with actinide ions via ion-exchange complexation with P-O− at the pH range, and complexation of neutral nitrate actinide salt with P=O at higher HNO3 concentration. The quantitative sorption of Pu(IV) at a HNO3 concentration could be attributed to the ability of Pu4+ ions to coordinate with nitrate ions and form a neutral complex with P=O group. The trivalent actinide ions such as Am3+ ions bind with phosphate groups via ion-exchange mechanism and, therefore, sorbs in a pH range. The sorption Am3+ ions was higher than UO22+ ions at given pH as it has higher charge than UO22+ ions and ion-exchange is highly dependent on the charges. It was interesting to note that UO22+ ions did not sorb at higher HNO3 concentration. This suggested that there was no stable complex formation of UO22+ with P=O group due to unsatisfied coordination with nitrate ions. The geometrical constraint did allow UO22+ ions to bind with multiple P=O groups to satisfy their coordination numbers. Therefore, the Ph-g-T substrate developed in the present work exhibited remarkable selectivity towards Pu(IV) ions that are known for forming stable complex with nitrate ions.
However, UO22+ was found to sorb quantitatively from seawater and ground water (88 ± 2%) and thus can be used for preconcentration of UO22+ ions from these aqueous matrices. The attainment of optimum uptake of Pu4+ ions in the Ph-g-T from 3 mol L−1 HNO3 solution as a function of time was studied, and observed that the Pu4+ uptake reached optimum value (92 ± 2%) in 4.5 h, and remained constant thereafter. The Pu4+-ions preconcentrated in the Ph-g-T could be de-loaded after two equilibrations with 0.2 mol L−1 hydrazine hydroxyl amine in 1 mol L−1 HNO3.
The Pu4+-uptake efficiency of Ph-g-T substrate must not expected to vary over a wide concentration range if the calibration plot or standard comparison method has to be used for the quantification. Therefore, the calibration plots were constructed by equilibrating the Ph-g-T substrates in the solutions having varying Pu4+ concentration (4-222 Bq mL−1). These Pu4+-loaded samples were subjected to alpha tracks analysis by exposing these to CR-39 for a fixed period of time. It is evident from the calibration plot given in Fig. 7 that the alpha track densities in CR-39 varied linearly with the amount of Pu4+ ions in the equilibrating solution. Similar observation was made using the fission track analysis. This suggests that the uptake efficiency remained constant over the concentration range studied.
Variations of alpha and fission track densities recorded in CR-39 and Lexan, respectively, exposed to different concentrations of Pu4+ ions loaded in Ph-g-T (1 × 2 cm2). CR-39 exposure time was kept 30 min, and fission tracks were recorded by exposing to neutron flux of order of 4 × 107 n cm−2 s−1 for 2 h
Ph-g-T was studied for its applicability in the estimation of uranium in water sample of natural origin using the fission track measurement. Ph-g-T (1 X 1 cm2 size) was equilibrated with 5 mL of sample of natural water and U standard solutions for overnight. The fission track densities of samples were correlated with the standards. The concentration obtained for two samples were 42.9 ± 2.3 and 252.3 ± 21.2 ng g−1 which were in a good agreement with that obtained by LASER fluorometric measurements i.e. 40 and 249 ng g−1, respectively. As SSNTD is more sensitive, the Ph-g-T based SSNTD method could be used for quantifying ppb level concentration (2–100 ppb) of UO22+ ions in the natural water as observed from the calibration plots.
For radionuclide derived from fission molly for medical objective, the total alpha activity in nano-Curie range has to be determined in the presence of curie level β-activity. In these cases, the SSNTD are best suited as β-particles do not form tracks and there is no possibility of electronic noise for a long exposure of detectors to accumulate sufficient alpha tracks. The easiest way is to immerse CR-39 detector in solution for desirable period (15–30 days) to record the alpha tracks. However, the alpha tracks registration efficiency in the solution is very low (Kwet = 5 × 10−4 cm−1, unit of efficiency is from balancing the dimension in equation = Td (track density)(tracks cm−2)/N λ (activity concentration in Bq cm−3) × t (exposure time in s)) as compared to thin solid source [33]. This is due to shorter range of alpha particles in the solution medium. Therefore, most of the alpha particles do not reach to the detector with sufficient energy to form the tracks. Only those alpha particles emitted by radionuclides in a close proxmity of the detector form tracks. This is evident from the images given in Fig. 8 where high alpha track density is seen in CR-39 exposed to alpha activity preconcentrated in the Ph-g-T with respect to CR-39 immersed in the 4.5 mL solution of Sr90–Y90 having 0.1 mol L−1 HCl. For the exposure time of 21.70 days of CR-39 in solution, the track density was 230 tracks cm−2 day−1. For the exposure time of 15.92 days of CR-39 kept on Ph-g-T in 2π contact, the track density was 59,883 tracks cm−2 day−1. The alpha activity determined in this sample was determined to 1.04 Bq mL−1 by standard comparison method having known alpha activity of mixPu (0.5–1.0 Bq mL−1). It is seen from Fig. 8b that < 0.10 Bq mL−1 alpha activity could be easily quantified by using this method.
Conclusions
Teflon was grafted with thin GMA polymer layer by mutual grafting technique and epoxide groups of poly (glycidyl methacrylate) were chemically modified to phosphate functionality. Teflon substrate with phosphate functionality was found to be selective towards Pu(IV) ions at a higher concentration of nitric acid (3–8 mol L−1). It was demonstrated that the Ph-g-T substrate could be used for Pu(IV) ions quantification in the aqueous samples by chemically selective alpha or fission tracks registration based SSNTD. The selective preconcentration of Pu(IV) would also enhance the detection limit to sub-ppb conc. Using this method, nano-Curie alpha activity in the 90Sr–90Y sample, having curie level of beta activity for medical objective, was quantified. U(VI) was found to preconcentrate in the phosphate group anchored Teflon from natural water, and used for its quantification using fission tracks based SSNTD.
References
Vajda N, Kim CK (2011) Determination of transuranium isotopes (Pu, Np, Am) by radiometric techniques: a review of analytical methodology. Anal Chem 83:4688–4719
Qiao J, Hou X, Mirób M, Roos P (2009) Determination of plutonium isotopes in waters and environmental solids: a review. Anal Chim Acta 652:66–84
Chamizo E, Jiménez-Ramos MC, Wacker L, Vioque I, Calleja A, García-León M, García-Tenorio R (2008) Isolation of Pu-isotopes from environmental samples using ion chromatography for accelerator mass spectrometry and alpha spectrometry. Anal Chim Acta 606:239–245
Guérin N, Calmette R, Johnson T, Larivière D (2011) Multi-dimensional extraction chromatography of actinides for alpha and mass spectrometry. Anal Methods 3:1560–1567
Labrecque C, Whitty-Léveillé L, Larivière D (2013) Cloud point extraction of plutonium in environmental matrixes coupled to ICPMS and α-spectrometry in highly acidic conditions. Anal Chem 85:10549–10555
Steinhauser G (2014) Fukushima’s forgotten radionuclides: a review of the understudied radioactive emissions. Environ Sci Technol 48:4649–4663
Paul S, Pandey AK, Kumar P, Kaity S, Aggarwal SK (2014) Tailored bifunctional polymer for plutonium monitoring. Anal Chem 86:6254–6261
Addleman RS, O’Hara MJ, Grate JW, Egorov OB (2005) Chemically enhanced alpha-energy spectroscopy in liquids. J Radioanal Nucl Chem 263:291–294
Karamanis D, Ioannides KG, Stamoulis KC (2006) Determination of 226Ra in aqueous solutions via sorption on thin films and α-spectrometry. Anal Chim Acta 573–574:319–327
Paul S, Pandey AK, Shah RV, Aggarwal SK (2015) Chemically selective polymer substrate based direct isotope dilution alpha spectrometry of Pu. Anal Chim Acta 878:54–62
Hanson SK, Mueller AH, Oldham WJ Jr (2014) Kläui ligand thin films for rapid plutonium analysis by alpha spectrometry. Anal Chem 86:1153–1159
Mannion JM, Locklair WD, Powell BA, Husson SM (2016) Alpha spectroscopy substrates based on thin polymer films. J Radioanal Nucl Chem 307:2339–2345
Locklair WD, Mannion JM, Husson SM, Powell BA (2016) Uptake of plutonium on a novel thin film for use in spectrometry. J Radioanal Nucl Chem 307:2333–2338
Mhatre AM, Chappa S, Paul S, Pandey AK (2017) Phosphate-bearing polymer grafted glass for plutonium(IV) ion-selective alpha spectrometry. J Anal At Spectrom 32:1566–1570
Rim JH, Armenta CE, Gonzales ER, Ünlü K, Peterson DS (2016) Evaluating bis(2-ethylhexyl) methanediphosphonic acid (H2DEH[MDP]) based polymer ligand film (PLF) for plutonium and uranium extraction. J Radioanal Nucl Chem 307:2327–2332
Duval CE, Darge AW, Ruff C, DeVol TA, Husson SM (2018) Rapid sample preparation for alpha spectroscopy with ultrafiltration membranes. Anal Chem 90:4144–4149
Sodaye S, Tripathi R, Pandey AK, Reddy AVR (2004) Scintillating polymer inclusion membrane for preconcentration and determination of α-emitting actinides. Anal Chim Acta 514:159–165
Chavan V, Agarwal C, Pandey AK (2016) Pore-filled scintillating membrane as sensing matrix for α-emitting actinides. Anal Chem 88:3796–3803
Love SF, Filby RH, Glover SE, Kathren RL, Stuit DB (1998) Use of combined alpha-spectrometry and fission track analysis for the determination of 240Pu/239Pu ratios in human tissue. Radioanal Nucl Chem 234:189–193
Hassan NM, Hanafy MS, Naguib A, El-Saftawy AA (2017) Controlling alpha tracks registration in Makrofol DE 1-1 detector. Nucl Instrum Methods Phys Res B 407:230–235
Zorri V, Remetti R, Capogni M, Cotellessa G, Falcone R (2017) Feasibility study on the application of solid state tracks detectors for fast surveys of residual alpha contamination in decommissioning activities. Radiat Meas 107:111–114
Misdaq MA, Aitayoub A, Chaouqi A (2018) Analysis of 238U, 232Th, 222Rn, and 220Rn in fresh and canned marine fish samples using solid state nuclear track detectors and resulting alpha radiation doses to adult consume. Health Phys 114:436–449
Chavan V, Paul S, Pandey AK, Kalsi PC, Goswami A (2013) Thin extractive membrane for monitoring actinides in aqueous streams. J Hazard Mater 260:53–60
Mhatre AM, Chappa S, Chavan V, Pandey AK (2017) Thin film of poly(bis[2-(methacryloyloxy)ethyl]phosphate) grafted on surface of poly(ether sulfone) membrane for plutonium(IV)-selective alpha tracks registration in CR-39 detector. J Radioanal Nucl Chem 314:187–196
Chappa S, Singha Deb AK, Ali SM, Debnath AK, Aswal DK, Pandey AK (2016) Change in the affinity of ethylene glycol methacrylate phosphate monomer and its polymer anchored on a graphene oxide platform toward uranium(vi) and plutonium(iv) ions. J Phys Chem B 120:2942–2950
Chappa S, Das S, Debnath AK, Sahu M, Saxena MK, Pandey AK (2016) spacer monomer in polymer chain influencing affinity of ethylene glycol methacrylate phosphate toward UO2 2+ and Pu4+ ions. Ind Eng Chem Res 55:8992–9002
Goel NK, Kumar Virendra, Pahan S, Bhardwaj YK, Sabharwal S (2011) Development of adsorbent from Teflon waste by radiation induced grafting: equilibrium and kinetic adsorption of dyes. J Hazard Mater 193:17–26
Chaudhari CV, Guin JP, Dubey KA, Bhardwaj YK, Varshney L (2016) Radiation induced grafting of glycidyl methacrylate on teflon scrap for synthesis of dual type adsorbent: process parameter standardization. Environ Prog Sustain Energy 35:1367–1373
Kalal J, Švek F, Marousek V (1974) Reaction of epoxide groups of glycidyl methacrylate copolymer. J Polym Sci Symp 47:155–166
Qiao B, Demars T, Olverade la Cruz M, Ellis RJ (2014) How hydrogen bonds affect the growth of reverse micelles around coordinating metal ions. J Phys Chem Lett 5:1440–1444
Chavan V, Patra S, Pandey AK, Thekkethil V, Iqbal M, Huskens J, Sen D, Mazumder S, Goswami A, Verboom W (2015) Understanding nitric acid-induced changes in the arrangement of monomeric and polymeric methacryloyl diglycolamides on their affinity toward f-element ions. J Phys Chem B 119:212–218
Iqbal MS, Jamil Y, Kausar T (2009) Thermal degradation study of glycidyl methacrylate acrylonitrile copolymers. J Therm Anal Calorim 96:225–233
Iyer RH, Chaudhuri NK (1997) Development of the track registration technique in solid state nuclear track detectors from solution media and its applications. Radiat Meas 27:529–548
Acknowledgements
Authors are thankful to Dr P. K. Pujari, Associate Director, RC&I Group, BARC for his keen interest and continuous encouragement in the present work.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Mhatre, A.M., Chappa, S., Chaudhari, C.V. et al. Phosphate functionalized radiation grafted Teflon for capturing and quantifications of U(VI) and Pu(IV) ions at ultra-trace concentration in aqueous samples. J Radioanal Nucl Chem 317, 1141–1149 (2018). https://doi.org/10.1007/s10967-018-5950-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-018-5950-x