Abstract
This paper describes a new analyte extraction medium called polymer ligand film (PLF) that was developed to rapidly extract radionuclides. PLF is a polymer medium with ligands incorporated in its matrix that selectively and quickly extracts analytes. The main focus of the new technique is to shorten and simplify the procedure for chemically isolating radionuclides for determination through alpha spectroscopy. The PLF system was effective for plutonium and uranium extraction. The PLF was capable of co-extracting or selectively extracting plutonium over uranium depending on the PLF composition. The PLF and electrodeposited samples had similar alpha spectra resolutions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A terrorist attack using nuclear device has been a great concern since the fall of the Soviet Union [1–5]. There had been numerous incidents where large quantities of nuclear materials were trafficked by terrorist from the former Soviet Union countries [1, 6, 7]. These materials were most likely diverted with intended use as a weapon. In event of a terrorist nuclear attack, an accurate and fast determination of the activity of radionuclides in a sample is critical to implicate responsible parties and form a response in a timely manner. Also, it is critical to have a high sample throughput since there is a potential need to analyze an enormous number of samples in a short time [1, 8]. Current radioanalytical techniques to analyze alpha emitting samples are quite mature and well established; however, they are slow and require highly trained personnel to perform extensive radiochemical separations and purification prior to analysis [8, 9]. These techniques are not well suited for rapid analysis or pre-screening of samples to determine which might be best suited for performing a more accurate but time-consuming set of analyses. Also, these classical methods require a fully functional chemistry laboratory to perform analyte separation to process the samples, which greatly limits the possibility of field analysis. These limitations of classical procedures greatly hinder the ability to accurately assess and respond to an incident in a prompt manner.
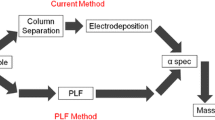
One of the possible solutions to improve the classical technique is to utilize ligands to combine separation and plating steps into a single step. The thin film extraction technique is similar to resin based extraction with the added benefit of an easier path forward for radiometric analysis for the alpha emitting elements. Selective extraction of analytes using a thin film substrate had been reported by several authors [10–19]. Oldham and his group synthesized Klaui ligand and used it to produce thin films to extract plutonium [14]. Plutonium recovery was high with Klaui ligand and alpha spectroscopy resolution was very good at ~33 keV. Surbeck has used commercially available resin beads to prepare thin films for uranium extraction. The films were prepared from finely ground resin beads, and the fine powder was fixed onto a flat surface. Fifty percent of uranium was recovered within 4 h, and 80 % was extracted in about 20 h [17]. The alpha spectroscopy peak resolution was not as good as the electrodeposited samples, probably due to the unevenness of the film surface. Wang et al. used a 54 mm2 Aliquat-366/PVC liquid membrane system to extract Cd(II) from an HCl solution [19]. The membrane was prepared by dissolving commercially available Aliquat-366 and PVC in THF then poured into a mold.
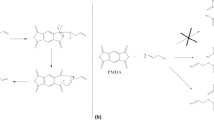
Our research group has extensively studied the possibility of using commercially available ligands to rapidly extract radionuclides [10, 12, 13]. Only commercially obtainable ligands were investigated since these ligands simplified the PLF manufacturing process by eliminating ligand synthesis. Also, it was more cost effective and provided consistency in a large scale batch. These ligands were formed into a polymer thin film similar to the ones used by Wang [19]. Di(2-ethyl hexyl) phosphoric acid (HDEHP) and bis(2-ethylhexyl) methanediphosphonic acid (H2DEH[MDP]) ligands were both examined for radionuclide extraction in a PLF form. H2DEH[MDP], which contains the diphosphonic group, is known to effectively retain alkaline earth metals and actinides, particularly for tetra and hexavalent oxidation states [20, 21]. Diphosphonic acids form strong complexes with metal ions through ionized phosphonic acid groups and P=O groups [22]. The chemical structure of bis(2-ethylhexyl) methanediphosphonic acid (H2DEH[MDP]) is shown in Fig. 1. In previous H2DEH[MDP] studies, several extraction conditions were examined to find an optimal condition for plutonium and americium extraction. H2DEH[MDP] based PLF was effective in extracting plutonium and americium from the 0.1 M nitric acid solution [12].
Several polymers were examined as a structure backing for ligands, and polystyrene has shown the best combination of analyte recovery and alpha spectra resolution [13]. The resolution of H2DEH[MDP] + polystyrene PLF was consistently better than PLFs based on nitrocellulose and poly(propylene) filter. Although many studies had been carried out on H2DEH[MDP] PLF, our group had not performed an in-depth extraction dependency study on solution acidity. H2DEH[MDP] ligand is extremely effective for actinide separation in a wide range of nitric acid concentration in resin bead form [23]. However, the extraction behavior may differ in PLF than in resin beads, and it is essential to find the best nitric acid concentration for analyte extraction.
Multiple PLFs were prepared on stainless steel substrates and used to test for plutonium and uranium extraction capability of the PLF system. The optimum analyte extraction conditions were found by changing the nitric acid concentration in tracer solution and the amount of extractants in PLF. Analyte extraction dependency on an equilibration time was also examined to optimize the exposure time. All the samples prepared in this experiment were examined using alpha spectroscopy, and high quality alpha spectra were obtained with minimal sample preparation steps.
Experimental
Materials
Bis(2-ethylhexyl) methanediphosphonic acid (H2DEH[MDP]) was obtained from Eichrom Technology Inc. No further purification was done to the ligands. Aqueous solutions were prepared using nitric acid from Fisher Scientific, and ultrapure deionized water was obtained from Barnstead Fi-Stream II Glass Still purification system. Tetrahydrofuran (THF) was obtained from Acros Organics. Polystyrene beads were obtained from Sigma-Aldrich. Polystyrene beads were not cross linked and density was 1.05 g/mL. 239Pu tracer and natural uranium were obtained from Eckert & Ziegler Isotope Products Inc.
Alpha spectroscopy
An Octet Plus system from Ortec, equipped with 900 mm2 ion implanted silicon detectors, was used in the entire experiment performed in this study. The manufacturer’s rated resolution for the detectors was 27 keV FWHM for 241Am at 5.486 MeV energy. Each detector was for calibrated energy and efficiency using a secondary NIST traceable source. Samples were counted on the top shelf, 4 mm away from the detector surface, for a minimum of 1440 min each to measure plutonium activity. Alpha spectroscopy data was analyzed using Bortels’ single tail alpha peak fitting algorithm [24].
PLF preparation and experimental conditions
Polymer ligand films were prepared by incorporating H2DEH[MDP] in the polystyrene structure. The stock solution was prepared by dissolving the ligands and the polystyrene beads in THF. Once the stock solution was prepared, it was directly deposited onto a 40 mm diameter stainless steel substrate. The deposited solution was air dried at room temperature overnight to evaporate THF and form a solid film. PLFs prepared with solvent casting deposited about 220 mg of film after evaporation of THF. The physical appearance of the PLFs changed depending on the amount of ligand in the film. The polystyrene used is clear in its natural form and the ligand is the only component causing the color change. Typically the films become more opaque with increasing ligand mass. An image of the PLFs is shown in Fig. 2, where (a) has larger amount of ligand used compared to (b). More detailed PLF preparation method was discussed in detail previously 10–12. Five PLF compositions were tested to find the optimum PLF for plutonium extraction. The PLF composition is described as the ratio between ligand and the entire solid mass. For example, PLF with one part ligand and one part polystyrene was assigned 1:2 (w/w) ratio. The ratios tested in this experiment were 1:5, 1:10, 1:15, 1:20 and 1:25 (wt/wt). 1:2 PLF was also prepared for the study, but it did not form a solid film and was excluded from any testing. The amount of polystyrene was kept constant in all four ratios but the mass of ligand in the solution was adjusted.
H2DEH[MDP] PLFs were tested over 0.01–8 M nitric acid solutions to generate a plutonium extraction performance. 239Pu solutions used in this study were prepared with 0.01, 0.1, 1, or 8 M nitric acid solution. Plutonium tracer solution was first dried on a hot plate then re-dissolved in a concentration adjusted nitric acid solution. Plutonium activity per volume was approximately 3 dpm/mL. For the PLF testing, 2.5–3 mL 239Pu tracer was directly stippled on the PLF surface, allowing the analyte to equilibrate for 3 h before removing the solution. The solution volume was selected to cover the entire PLF surface. Some of the tracer solution evaporated during the equilibration time and 1–2 mL of solution was remaining on the PLF substrate after 3 h. After removing the tracer solution, PLFs were thoroughly rinsed with deionized water to remove any nitric acid remaining on the surface and to remove any tracer that was not bound to the surface. PLFs were then allowed to air dry to remove any water that may have been left on the polymer medium. The plutonium activity of each sample was measured by direct alpha counting to quantify the plutonium recovery by H2DEH[MDP] PLF.
Results and discussion
The plutonium recovery by H2DEH[MDP] PLF showed a dependency both on the nitric acid concentration and the composition of the polymer film. H2DEH[MDP] PLF was able to extract plutonium in all nitric acid concentrations tested as shown in Fig. 3. 1:10, 1:15, and 1:20 PLFs were all effective in plutonium extraction from 0.01 to 1 M nitric acids. The highest recovery for these PLFs all occurred at 1 M tracer solution. The percent recoveries were 50.44 ± 8.27 and 47.61 ± 7.17 for 1:10 and 1:20 PLF, respectively.
The plutonium recovery for 1:5 PLF was noticeably lower than the other PLFs from 0.01 to 1 M. However, the recovery was higher at 8 M than other PLF types tested. 1:25 PLFs were also tested but those were unstable and showed a tendency to develop bubbles while in the vacuum chamber of the alpha spectroscopy system. About 90 % of the 1:25 PLFs developed bubbles, and in some cases the polymer film shattered into pieces. The bubbles were believed to have been caused by gas trapped in the polymer structure, most likely THF. Larger ligand content in the PLF is believed to provide more porous surface for gas to escape from the polymer structure. In the 1:25 PLF, which contained the lowest amount of ligand, large amount of gas was being trapped during the PLF synthesis due to inadequate venting. Once vacuum was applied, the trapped gas in the PLF expanded and caused a ballooning effect on the surface as it escaped from the polymer structure. Due to the stability issue, 1:25 data was not included in Fig. 3.
It was expected for 1:5 H2DEH[MDP] PLF to have the highest recovery due to having a higher number of ligands presented in the PLF compared to the other PLF compositions tested. The ligand is the only component within the PLF to have any significant affinity to plutonium, and the more ligands meant more binding sites for 11Pu. This result clearly showed that the plutonium extraction was not only dependent on the amount of ligand presented in the PLF but many other factors, such as ligand orientation, ligand complexation, and plutonium oxidation state. Plutonium has five oxidation states, and up to four different oxidation states can co-exist in a solution [25]. It was impossible to measure the plutonium oxidation states in the solutions used in the experiment due to low plutonium quantity in each solution. However, it is suspected that both +3 and +4 oxidation states co-exist in the tracer solution [25]. The H2DEH[MDP] have shown effectiveness in both Pu(III) and Pu(IV). The H2DEH[MDP] ligands are theorized to form various length complexes with each other as the PLF is synthesized and plutonium extraction behavior changes based on the length of the complex. This means that certain complexes are only effective for Pu(III) extraction, and the other complexes are only effective for Pu(IV). More ligands in the stock solution seems to cause H2DEH[MDP] to form complexes that are mostly effective for Pu(IV) extraction. As the amount of ligand in the stock solution decreases, two distinctive ligand complexes form, one for Pu(III) and the other for Pu(IV). Another possible explanation for the plutonium extraction behavior observed is that nitric acid is changing the orientation of ligands to be more favorable for plutonium extraction at certain nitric acid concentrations. For example, ligands in 1:10, 1:15, and 1:20 PLFs are oriented more favorably for plutonium extraction at 0.1 or 1 M nitric acid.
The quality of alpha spectra obtained from the PLF system was compared to one from electrodeposited sample. Figure 4 was plotted with normalized count data from PLF and electrodeposited samples. Both spectra had similar resolution and tailing characteristics. 1:10, 1:15, and 1:20 PLFs were all suitable for plutonium extraction. Out of three compositions, 1:20 used the least amount of H2DEH[MDP] ligands to manufacture PLFs, which makes it more cost effective.
The equilibration time of 3 h was used to generate a baseline for plutonium extraction behavior for the H2DEH[MDP] PLF. The time was chosen to provide enough time for ligands to form complexes with the plutonium. However, the PLF method is being developed to rapidly process samples, and it is a key to have the shortest equilibration time possible. It is important to examine plutonium extraction dependency on equilibration time to decrease analysis time. In this experiment, 1:20 H2DEH[MDP] PLF was tested with 0.1 M nitric acid. The extraction condition was kept consistent throughout the experiment except for the exposure time. The exposure times used in this experiment were from 10 to 180 min. The plutonium recovery linearly increased from 10 to 90 min exposure time then started to level off after 90 min exposure time as shown in Fig. 5. The maximum plutonium recovery of 44 % was achieved at 180 min equilibration time. However, the standard deviation at 180 min exposure time was larger than other measurements. 90 and 120 min recoveries were within the standard deviation of the 180 min recovery. Student’s t-test was performed to confirm and assess the statistic difference between plutonium recoveries between 90 and 180 min. The recoveries measured at 90 and 120 min exposure time was statistically indifferent from the 180 min measurement at 95 % confidence level.
The most important aspect that can be gathered from the time study is that the PLF was able to extract plutonium even at 10 min equilibration time. The recovery was only slightly higher than 10 %; however, even 10 % may provide sufficient activity to perform a radiometric analysis depending on the sample activity. In a post-detonation situation, sample activity near ground zero is expected to be high enough for even a very short equilibration time to extract enough plutonium for a radiometric analysis. In the case of environmental samples, which typically have low activity, 10 % recovery would likely only allow qualitative analysis using radiometric techniques. However, if the PLF technique is only used as a screening method before performing a more precise analysis, such as mass spectroscopy, a qualitative analysis will provide adequate information to select critical samples and shorten the total analysis time.
H2DEH[MDP] was designed for an actinide group separation and also showed high affinity for uranium. Since uranium alpha spectra peaks are well separated from plutonium peaks, it is possible to co-extract plutonium and uranium onto PLF then perform alpha spectroscopy to qualify. PLFs were examined for uranium extraction using a natural uranium tracer. The condition tested for uranium extraction was the same as the baseline plutonium experiment; 1:5, 1:10, and 1:20 H2DEH[MDP] PLFs were tested over 0.01–8 M nitric acid solutions. The uranium extraction behavior was entirely different than the plutonium extraction. Neither 1:10 nor 1:20 PLF was effective in uranium extraction over all nitric acid ranges tested. 1:5 PLF showed the highest recovery of ~30 % with 1 M nitric acid as shown in Fig. 6. Also, about 22.5 % of uranium was extracted using 1:5 PLF at 0.1 M nitric acid. Data shows that H2DEH[MDP] PLF can be used to selectively extract plutonium over uranium or simultaneously extract uranium and plutonium by changing the composition of the PLF. For example, with 1:5 PLF, uranium can be co-extracted along with plutonium at 0.1 or 1 M nitric acid. At the same nitric acid concentration, 1:20 PLF can be used to extract plutonium over uranium.
The analyte selectivity based on PLF composition was further verified in the co-extraction experiment by using mixed uranium and plutonium tracer solution. The mixed tracer solution was prepared by drying 239Pu and natural uranium then re-dissolved in 1 M nitric acid. The standard PLF testing procedure was used with the mixed tracer solution. 4.95 dpm of plutonium and 5.24 dpm of uranium were used to prepare each sample. The experiment confirmed that H2DEH[MDP] PLF is capable of co-extracting or selectively extracting plutonium over uranium depending on the PLF composition. Plutonium and uranium percent recovery by each PLF is shown in Fig. 7. 1:5 H2DEH[MDP]PLF simultaneously extracted 23 % of plutonium and 20 % uranium. However, neither 1:10 nor 1:20 PLFs showed any affinity to uranium and selectively extracted plutonium over uranium.
Conclusions
The PLF method is a great screening tool to deploy to decrease the number of samples required for more extensive analysis. The entire sample preparation to analysis was done within one to 2 days. Compared to the PLF method, the classical method for alpha samples takes 2 days to a week. The exact analysis time may vary as the counting time may have to be adjusted depending on the sample activity. The technique also requires minimal chemicals, and it is field deployable. The reduction in time and simplified procedure make this technique ideal for the post-detonation emergency response.
H2DEH[MDP] PLFs were effective in plutonium and uranium extraction. 1:10, 1:15, and 1:20 PLFs showed similar plutonium extraction behavior. Since 1:20 H2DEH[MDP] PLF was most cost effective, most experiments were performed with 1:20 PLFs. Close to 50 % of plutonium was extracted by 1:20 PLF with 1 M nitric acid. H2DEH[MDP] PLF showed consistency similar to the electrodeposited samples. The overall analyte recovery was lower than the electrodeposited samples. However, PLF is designed to be a rapid field deployable screening technique, and consistency is more important than the recovery. H2DEH[MDP] PLF was capable of co-extracting or selectively extracting plutonium over uranium depending on the PLF composition. With 1:5 PLF, about 23 % of plutonium and 20 % uranium were simultaneously extracted with 1 M nitric acid. 1:10 and 1:20 PLFs preferably extracted plutonium over uranium with 1 M nitric acid. The uranium alpha spectra peaks were well separated from the plutonium peaks, and it was possible to perform isotopic measurements.
References
Helfand I, Forrow L, Tiwari J (2002) Nuclear terrorism. BMJ 324:356–359
Grant PM, Moody KJ, Hutcheon ID et al (1998) Nuclear forensics in law enforcement applications. J Radioanal Nucl Chem 235:129–132. doi:10.1007/BF02385950
Mayer K, Wallenius M, Fanghänel T (2007) Nuclear forensic science—from cradle to maturity. J Alloys Compd 444–445:50–56. doi:10.1016/j.jallcom.2007.01.164
Mayer K, Wallenius M, Ray I (2005) Nuclear forensics—a methodology providing clues on the origin of illicitly trafficked nuclear materials. Analyst 130:433–441. doi:10.1039/B412922A
Press release (2014) Calculating the new global nuclear terrorism threat. http://www.iaea.org/newscenter/pressreleases/2001/nt_pressrelease.shtml. Accessed 9 Sept 2015
Baker H, Cutler L (2000) A report card on the department of energy’s nonproliferation programs with Russia. United States Department of Energy
United Nations Audiovisual Library of International Law (2014) http://legal.un.org/avl/ha/icsant/icsant.html. Accessed 9 Sept 2015
Pöllänen R, Siiskonen T (2006) High-resolution alpha spectrometry under field conditions–fast identification of alpha particle emitting radionuclides from air samples. J Environ Radioact 87:279–288. doi:10.1016/j.jenvrad.2005.12.004
Plionis AA, Rim JH, Hastings EP et al (2009) Micro-electrodeposition techniques for the preparation of small actinide counting sources for ultra-high resolution alpha spectrometry by microcalorimetry. J Radioanal Nucl Chem 282:905–908. doi:10.1007/s10967-009-0251-z
Rim JH, Gonzales ER, Armenta CE et al (2013) Developing and evaluating di(2-ethylhexyl) orthophosphoric acid (HDEHP) based polymer ligand film (PLF) for plutonium extraction. J Radioanal Nucl Chem 296:1099–1103. doi:10.1007/s10967-012-2266-0
Rim JH, Peterson DS, Armenta CE et al (2015) Evaluating ligands for use in polymer ligand film (PLF) for plutonium and uranium extraction. J Radioanal Nucl Chem. doi:10.1007/s10967-015-4118-1
Gonzáles ER, Peterson DS (2009) Rapid radiochemical sample preparation for alpha spectrometry using polymer ligand films. J Radioanal Nucl Chem 282:543–547. doi:10.1007/s10967-009-0218-0
Gonzáles ER, Klingensmith AL, Peterson DS (2011) Rapid separation and extraction of radioactive analytes onto filters and surfaces. Proc Radiochem Suppl Radiochim Acta 1:194–200. doi:10.1524/rcpr.2011.0035
Hanson SK, Mueller AH, Oldham WJ Jr (2014) Kläui ligand thin films for rapid plutonium analysis by alpha spectrometry. Anal Chem 86:1153–1159. doi:10.1021/ac402997e
Oldham WJ, Dry DE, Mueller AH (2009) Synthesis of functional monolayer surfaces for rapid radiometric determination of plutonium. J Radioanal Nucl Chem 282:585–589. doi:10.1007/s10967-009-0243-z
Koulouridakis PE, Kallithrakas-Kontos NG (2004) Selective mercury determination after membrane complexation and total reflection X-ray fluorescence analysis. Anal Chem 76:4315–4319. doi:10.1021/ac049780a
Surbeck H (2000) Alpha spectrometry sample preparation using selectively adsorbing thin films. Appl Radiat Isot 53:97–100
Graul TW, Li M, Schlenoff JB (1999) Ion exchange in ultrathin films. J Phys Chem B 103:2718–2723. doi:10.1021/jp983049o
Wang L, Paimin R, Cattrall RW et al (2000) The extraction of cadmium(II) and copper(II) from hydrochloric acid solutions using an Aliquat 336/PVC membrane. J Membr Sci 176:105–111. doi:10.1016/S0376-7388(00)00436-1
Horwitz EP, Chiarizia R, Dietz ML (1997) DIPEX: a new extraction chromatographic material for the separation and preconcentration of actinides from aqueous solution. React Funct Polym 33:25–36. doi:10.1016/S1381-5148(97)00013-8
Moyer BA (2009) Ion exchange and solvent extraction: a series of advances, vol 19. Taylor and Francis, Hoboken
Herlinger AW, Ferraro JR, Chiarizia R, Horwitz EP (1997) An investigation of P, P′-di(2-ethylhexyl) methanediphosphonic acid and some of its metal complexes. Polyhedron 16:1843–1854. doi:10.1016/S0277-5387(96)00495-0
Siemann U (2005) Solvent cast technology—a versatile tool for thin film production. Springer, Berlin, pp 1–14
Bortels G, Collaers P (1987) Analytical function for fitting peaks in alpha-particle spectra from Si detectors. Int J Rad Appl Instrum [A] 38:831–837. doi:10.1016/0883-2889(87)90180-8
Katz JJ, Morss LR, Edelstein N, Fuger J (2011) The chemistry of the actinide and transactinide elements, 4th edn. Springer, Dordrecht
Acknowledgments
This research was performed with the support of the U.S. Departments of Energy Office of Nuclear Nonproliferation Research and Development and U.S. Department of Defense’s Defense Threat Reduction Agency. The authors also gratefully acknowledge the support from the Nuclear Forensics Graduate Fellowship Program which is sponsored by the U.S. Department of Homeland Security’s Domestic Nuclear Detection Office and the U.S. Department of Defense’s Defense Threat Reduction Agency. Los Alamos National Laboratory is operated by Los Alamos National Security, LLC for the U.S. Department of Energy under contract number DE-AC52-06NA25396. This document had been reviewed and assigned publication number: ‘LA-UR-14-24118’ Version 3.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rim, J.H., Armenta, C.E., Gonzales, E.R. et al. Evaluating bis(2-ethylhexyl) methanediphosphonic acid (H2DEH[MDP]) based polymer ligand film (PLF) for plutonium and uranium extraction. J Radioanal Nucl Chem 307, 2327–2332 (2016). https://doi.org/10.1007/s10967-015-4444-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4444-3