Abstract
This study investigated the status and distribution patterns of selected heavy metals in roadside soils along Irbid-North Shooneh Highway, Jordan. This highway has experienced a growing number of vehicles that are likely to influence the levels of heavy metals in the surrounding agricultural lands. The average concentrations of Cr, Co, Cd, Cu, Pb, Zn, and Ni were 16.0, 36.0, 11.0, 4.0, 79.0, 122.0, and 60.0 mg/kg, respectively. Cd, Pb, and Co showed average levels that are higher than the average world soil background values. Elevated levels of heavy metals were measured in surface soil layer which decreased with depth, and with distance from the roadway. The contamination factor (CF), pollution load index (PLI), single ecological risk (Ei), potential ecological risk index (PERI), and geo-accumulation index (Igeo) generally indicated that the roadside soils are contaminated with Cd, Pb, and Ni. Heavy metals in soils are of geogenic and anthropogenic origins. Weathering of parent rocks in Wadi Al-Arab catchment is the primary natural source, whereas agrochemicals, vehicle exhausts, degradation of surface wear and paint of vehicles, vehicle wear debris of tire, and brake lining are the main anthropogenic sources of heavy metals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the recent years, a large number of studies investigated the levels of contaminants associated with highways, of which heavy metals received particular interest (Christoforidis and Stamatis 2009; Wei and Yang 2010; Apeagyei et al. 2011; Moreno et al. 2013; Bretzel et al. 2014; Liu et al. 2014; Adamiec et al. 2016; Kim et al. 2016; Wang et al. 2017). In urban areas, heavy metals may come from different sources, of which vehicle emissions are the most important (Nazzal et al. 2013; Kelly et al. 1996). Elevated levels of metals have been found on street dusts and in soils bordering highways, with the contamination decreasing with distance from the road (Ellis and Revitt 1982; Fergusson et al. 1980; and Howari and Banat 2001).
Many of these metals are toxic to living organism, and even heavy metals considered micronutrients for life can be toxic when present at excessive levels that impair important biological processes and pose a threat to flora and fauna (Babula et al. 2008; Al-Taani et al. 2012; El-Radaideh et al. 2017b). They show a tendency to accumulate in soils (Howari et al. 2004) and in human body and pose harm to human health (Faiz et al. 2009; Al-Taani et al. 2013). USEPA (2002) has listed Cd, Co, Cr, Mn, Ni, and Pb as hazardous air pollutants, among them, Cd, Cr, and Ni have been included in the carcinogen classification (ATSDR 2007). Heavy metals of highways are easily re-suspended back into atmosphere as a result of changing in environmental and in climate conditions, so they can be described as “chemical time-bombs” (Ferreira-Baptista and De-Miguel 2005). Heavy metals have a long residence time because of the interactions with particular soil components (Charlesworth et al. 2003; Tian 2005).
In the urban environment, toxic elements are commonly found in highway dust which can potentially be harmful to roadside soils, vegetation, wildlife, and neighboring human settlements. In their study, Turer et al. (2001) have confirmed the mutuality of the close relationship between heavy metal concentration in topsoil and in the dust falls. Heavy metals have been reported to be released into the atmosphere through different mechanisms (Zhang et al. 2013; Sharma and Prasad 2010. Soils, vegetation, and food are major sinks for the atmospheric deposition (Rachwal et al. 2014).
The presence of heavy metals has been considered a useful indicator for contamination in surface soil, sediment, and dust environments (Ubwa et al. 2013; Al-Taani et al. 2015b). While heavy metal pollution of soils has gained a growing attention in Jordan, their status and impacts in urban roadside soils have not been fully investigated. This study examines the local background concentrations and distribution characteristics of selected heavy metals in agricultural soil along a major traffic highway in Irbid-North Shooneh. In addition, it evaluates the degree of contaminations and the potential sources of heavy metal contaminants in the region.
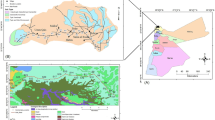
This study, North Shooneh, is located in Irbid Governorate, northwestern Jordan between 32° 33′20″N and 35°51′00″E, with an elevation of 570 m above sea level (asl). Irbid is the second largest city in Jordan with the highest population density of 1,770,000 in 2015. The study area, with a population of 122,330, is a major ground transportation center to Syria (Fig. 1). In addition, more than 10,000 vehicles use Irbid-North Shooneh highway each working day. This highway has experienced a growing number of vehicles, with increasing emissions that are likely to impact the surrounding agricultural lands.
The study area is a part of eastern Mediterranean weather regime with an annual rainfall total of about 500 mm and a dominant wind direction from west to northeast.
Geology and soil
The major geologic units exposed in the study area belong to the Upper Cretaceous and Tertiary. They consist of the following formations (in ascending order): Amman Silicified Limestone or Al Hisa Phosphorite, Muwaqqar Chalk Marl and Umm Rijam Chert Limestone. These units are mainly composed of calcareous depositions (limestone, dolomite, marl, and chalk) interrupted by siliceous facies and siliclastics, especially in Al Hisa Phosphorites (Bender 1968) (Fig. 2).
Geological map of the study area modified after Ibrahim and Al-Mashakbeh (2016)
IUSS WRB (2007) found that Vertic Cambisols and Vertisols are commonly observed on stable topography, whereas calcaric Cambisols and Lithosols are widespread on steep areas. Kraushaar (2016) further explained that variations in soils developed on slopes are dependent on the type and hardness of geologic parent material, with brown soil formed on consolidated rocks (Leptosols), and Cambi- or Regosols on slopes of less consolidated marly unit. Soils in the study area are fertile, with a high water-holding capacity and are suited for cereal crops (Khresat et al. 1998; Al-Qudah 2001).
Materials and methods
Twenty sampling sites were selected from areas surrounding Irbid-North Shooneh highway (which is approximately 30 km long). For each site, samples were taken from two depths 0–10 and 10–20 cm. The sampling sites were collected from both sides of the highway in the surrounding agricultural region of Wadi Al-Arab catchment. These sites are located at varying distances (from 0 to 40 m) off the highway (Figs. 1 and 2). Samples were collected using a stainless steel spatula and kept in plastic bags for 24 h. Due to difficulty in accessibility across the area, sampling sites were not uniformly distributed. The soils of Irbid–North Shooneh are suffering from serious pollution problems due to adjacent Irbid Industrial city, high population, and high traffic density. The highway was selected based on traffic load and anthropogenic activities. People living in the surroundings of the study area use their vehicles to move to work in nearby locations like Irbid, Koforyooba, Kuforased, North Shooneh, and West Bank (Fig. 1). The control samples were obtained from Koforyooba soil (Fig. 1) where soils are distant from human activities and uncultivated.
The collected samples were initially air-dried and sieved through a 2-mm plastic sieve for removal of large grains (Manta et al. 2002). Surface soil samples were characterized for grain size distribution using hydrometer method (Gee and Bauder 1986; Madrid et al. 2002). Fine fractions were wet-sieved through a 63-μm sieve to separate the mud from other fractions. The fine fraction (63 μm) was further analyzed with a Computerized Sedigraph Analyzer-2000. The clay-sized fraction was then separated from silt by the pipette method (Folk 1974; Loring and Rantala, 1992). Heavy metal concentrations in soil samples with a particle size of ≤ 2 μm were investigated using the pipette analysis method (Williams et al. 2000; El-Radaideh et al. 2014). In this method, the sample was pipetted at different times and various depths of the suspension of the sample in a measuring cylinder. The pipetted suspension was condensed and dried, and the mass ratio of the pipetted fraction was determined. Then, 0.5 g of the pipetted fraction was digested using 4 ml of 139 HNO3, conc. (65%), 2 ml of HF, conc. (40%), and 4 ml of HClO4, conc. (70%). The solution of the digested samples was analyzed using the standard methods of atomic absorption spectroscopy-model Philips SP 9PYE Unicam Spectrophotometer for Cr, Co, Cd, Cu, Pb, Zn, and Ni. For quality control, triplicates of each sample were analyzed, and average values were calculated. The accuracy of atomic absorption spectrometer analysis was checked using National Institute of Standards and Technology, standard reference material SRM 1646l 146. Correlation coefficients between soil variables were calculated with the SPSS 147 software package. Four oriented clay samples were prepared for XRD analysis to identify clay minerals in the very fine mud fractions (Grim 1968; Carroll 1970, Banat et al. 2005). Samples were examined untreated, ethylene glycolated, and heated at 600 °C (Fig. 4). X-ray diffraction analysis was conducted under the following conditions: 2θ 0–30°; X-ray radiation: Cu kα; generator current 30151 mA; generator voltage 30 kV; and scanning speed 0.05 cm/min at Yarmouk university. Bulk soil fractions were also analyzed by XRD for seven samples at Al Al-Bayt University (Appendix 1, Fig. 4).
Electrical conductivity (EC) in a 1:5 extract and calcium carbonate (CaCO3) equivalent were determined by a manometric method. Total organic matter (TOM) in each sample was measured by the titration method (Hesse 1972). The calciometry method (Loring and Rantala 1992) was used to determine the content of carbonate (CaCO3) in the soils, in which the amount of CO2 released from the reaction depends on the amount of CaCO3 in the sample. Analyses of trace metals were performed at the Department of Earth and Environmental Sciences, Yarmouk University, Jordan. The pH values of the selected soil samples were also determined using the method described by Allen et al. (1974). About 4.0 g of the soils (< 2 mm) was mixed with 10.0 ml of deionized water in centrifuge tubes. The mixtures were shaken for 30 min on a mechanical shaker and then centrifuged at 3000 rpm for 10 min. The pH of supernatants was measured using a pre-calibrated pH meter (Oakton Acron Series).
To be able to assess the intensity of anthropogenic contamination, and extent of pollution in topsoil of study area, it is necessary to establish the natural background value for the soils, and enrichment may then be well-defined as the difference between the current value of heavy metal and the geochemical background value. A quantitative measure of the extent of selected heavy metal pollution in the studied soils was calculated using the geo-accumulation index (Igeo) proposed by Muller (1981). Igeo has been used by various researchers (Loska et al. 2003; El-Radaideh 2016).
where Cn is the measured concentration of the examined element n in the soil, and Bn is the geochemical background concentration (or reference value) of the element n. Factor 1.5 is a background matrix correction factor for lithogenic effects. Constant 1.5 allows for analyzing the natural fluctuations in heavy metal content in the topsoil and to detect very small anthropogenic influence. The average level in Global Geochemical Background of Cambisols (GGBC) (silty and loamy soils) (Kabata-Pendias 2011), the average crustal abundance (Fortescue 1992), and the average geochemical background level in control samples (MGBLC) from Koforyooba sampling site (Fig. 1) were used as the background concentrations. The Igeo index consists of seven grades (0 to 6), indicating various degrees of enrichment above the background values and ranging from unpolluted to very highly polluted soil quality.
The degree of the pollution of the studied soils was assessed by the contamination factor (CF), which represents the individual impact of each metal on the soil (Hakanson 1980; Chen et al. 2005). The CF is the concentration of heavy metal divided by the background value (Hakanson 1980). Background values used here are the standards reported by Kabata-Pendias (2011) GGBC.
In order to evaluate the data in more detail, the pollution load index (PLI) was used, where the PLI of metals was calculated according to the following formula (Tomllinson et al. 1980; Salmons and Forstner 1984; Angulo 1996):
where CF is the calculated contamination factor, and n is the number of heavy metals (7 in the present study).
To assess the effect of multiple metal pollution in the studied soils, the formula developed by Hakanson (1980) was used. According to this formula, the potential ecological risk index (PERI) is defined as
where Ei is the single ecological risk index, and Ti is the toxic response factor for a given element (e.g., Cd = 30, Zn = 1, Pb = Ni = Co = Cu = 5, and Cr = 2) (Muller 1981).
CFi is the calculated contamination factor for the same metal, and the CF of each metal was classified as either low (CF < 1), moderate (1 ≤ CF > 3), considerable contamination (3 ≤ CF < 6), or very high contamination (CF ≥ 6) (Hakanson 1980).
Ei was classified as either low risk (Ei < 40), moderate (40 ≤ Ei < 80), considerable contamination (80 ≤ Ei < 160), high (160 ≤ Ei < 320), or significantly high risk (Ei ≥ 320) (Hakanson 1980). The PLI of each metal was classified as polluted (PLI > 1), baseline level (PLI = 1), or not polluted (PLI < 1) (Tomlinson et al. 1980).
The PERI consists of four grades: < 150 low, 150 ≤ PERI < 300 moderate, 300 ≤ PERI < 600 considerable, and ≥ 600 very high (Hakanson 1980).
Relationships between heavy metals and other soil factors were determined by Pearson coefficient in a two-tailed test (r < 0.01 and 0.05).
Results and discussion
Soil parameters
The granulometric analysis of soil samples (Fig. 3 and Table 1) showed that they were composed of 3.6% sand, 52.1% silt, and 44.4% clay, indicating predominance of silt and clay in the sampling sites with a clay–loam texture.
Soil samples were assessed by XRD analysis (Fig. 4). Similar mineralogical composition was generally observed in most of the analyzed samples, where calcite, quartz, dolomite, and clay minerals (kaolinite, illite, vermicullite, palygorskite, and montmorillonite) are dominated, with minor anorthite-feldspar and sepiolite clay.
X-ray powder diffractometer patterns of selected soil samples (collected from 0 to 10 cm depth) along Irbid-North Shooneh Highway, where a, b, and c represent the bulk sample and is for the fine fraction of < 63 μm. (Q: Quartz, Cal: Calcite, Dol: Dolomite, Palyg: Palygorskite, An: Anorthite (feldspar), Sep: Sepiolite, I: Illite, K:Kaolinite, M:Montmorillonite and V: Vermiculite
EC, pH, CEC, TOM, and carbonate contents of soil samples are tabulated in Table 1. The pH showed slightly neutral values and exhibited no vertical variations. In addition, no considerable spatial changes in pH levels in soil samples were observed along the selected highway. While the pH plays significant role in sorption and desorption of heavy metals in soil (Elliot et al. 1986; Batayneh and Al-Taani 2015), the measured values suggest that the impact of pH on desorption of heavy metals is largely insignificant. However, sample sites with soil pH value of 7.8 may enhance ionic retention (Kluge and Wessolek 2012). The relatively moderate pH values are due to the buffering effect of carbonate minerals (calcite and dolomite minerals). These minerals dominate the soils of the northern region of Jordan and are originated from weathering of carbonate rocks of Amman Silicified Limestone, Muwaqqar Chalk Marl, and Umm Rijam Chert Limestone. These units are mainly composed of calcareous depositions (limestone, dolostone, marl, and chalk) (Bender 1968) (Fig. 2).
This is consistent with the high CaCO3 content observed in soil samples ranging from 13.0 to 22.1%. The TOM contents of soil are low to medium (less than 2.1%). Soils closer to the road and park stations showed higher content of TOM than that of distant soils (sampling sites 1, 2, 19, and 20). Higher percent of TOM is probably resulted from vegetation debris, fuel combustion, and oil leakage. In addition, elevated levels of TOM were associated with fine soil fractions with a correlation coefficient between clay-sized fraction and TOM of 0.70 (Table 2), whereas lower values were found in the coarser fractions (sand). The CEC of topsoil ranged from 89.0 to 127 cmol(+)/kg with a mean value of about 113cmol(+)/kg decreasing vertically in soil profile to approach an average value of 44 cmol(+)/kg at 10–20 cm deep. The decrease in CEC values in subsoil is likely due to rooted plants, deficiency of organic debris, and the dominance of kaolinite clays (Meyer et al. 1994).
Elevated levels of EC were detected in samples collected close to sampling sites 1, 2, 3, 4, and 5. The variation in EC values can be attributed to the type of irrigation water, where Doqra untreated wastewater is commonly used for irrigation. The excessive use of pesticide and ammonic fertilizers may also explain the increase in EC (Abu-Rukah and Samawi 2000; Batayneh et al. 2014). In addition, the higher values can be attributed to wet climatic conditions during winter, which result in increased dust deposition with subsequent decrease in residence time of aerosols (Yusuf et al. 2011).
Heavy metals
The average concentrations of heavy metals in soil samples in relation to different background values are presented in Table 3. A wide range of concentrations was observed for each metal (Figs. 5 and 6).
In general, the average concentrations of heavy metals decreased vertically with soil depth (Fig. 5). Among others, this may be related to leaching and accumulation at greater depth (beyond the investigated depths), with lower content of sand compared to silt and clay at these sites. It is expected that heavy metals in soil will decrease with decreasing distance from Koforyooba control sites, though our data are not sufficient to give statistical validity to this suggestion. Li (2005) documented a slight depletion in the heavy metal contents in roadside soils with further distance from the highway.
Figure 6 shows higher levels of heavy metals at both ends of the sampling transect. These ends represent areas with frequent traffic jams and stop-start maneuvers (due to traffic lights), with subsequent higher concentrations of metal emissions and depositions. In addition, this can be attributed to the urban activities in Irbid and Shooneh cities which are located in the far eastern and western sides of the studied highway.
Lead (Pb)
The high concentration of Pb near sites 1 and 2 can be attributed to vehicle exhaust, where leaded gasoline is the predominant gasoline used in the study area. According to Abu-Rukah (2002), about 76% of petrol lead is emitted to the atmosphere by vehicle exhaust in the surroundings of the study area. More than 50% is falling as atmospheric deposition. The Pb levels in soil samples were higher than the world average soil concentration (WMS) of 35 mg/kg (Table 3), and higher than the soil around the Jordanian steel industry (Al-Khashman and Shawabkeh 2009) (Table 4 and Fig. 7). Odat (2013a, b) found that Pb concentration in soils along Irbid/Zarqa Highway, Jordan ranged between 18.51 and 79.99 mg/kg with an average of 47.95 mg/kg. Mashal et al. (2009) measured an average Pb concentration of 98.8 mg/kg in urban soils of Al-Hashimeyeh, central Jordan. Howari et al. (2004) recorded Pb content of 70 mg/kg in soils along Dead Sea-Aqaba Highway, southern Jordan (Table 4 and Fig. 7). The average Pb/Zn ratio in soil samples ranged between 1.6 (in soil depth of 0–10 cm) and 1.2 (from 10 to 20 cm). These ratios of more than one unit indicate unconfined contamination and pollution inputs, as well as auto-repair workshop wastes and exhaust emission by vehicles (Hewitt and Candy 1990). The correlation analysis showed that the concentrations of Pb in soil samples were significantly correlated with Zn, Cr, Co, Cd, Ni, and Cu (Table 2). This could be attributed to higher use of leaded gasoline and to higher dry deposition to arid climate in summer.
Map showing the locations of previously investigated urban topsoil in different parts of Jordan and are presented in Table 4
Cadmium (Cd)
Elevated levels of Cd were measured in soil samples ranging from 5 to 20 mg/kg with an average of 10.7 mg/kg, compared to the world average Cd content in soil of 0.53 mg/kg (Table 3). High content of Cd was also recorded in control samples (collected from agricultural farmlands, south of Koforyooba town, Fig. 1). North Shooneh area is well known for its intense agricultural activities with frequent application of fertilizers. Application of certain phosphoric fertilizers adds Cd and other potentially toxic elements to the soil, such as Pb (Mmolawa et al. 2011). This suggests that Cd in roadside soils is likely to originate from vehicle exhaust and intense application of P-fertilizers in the surrounding agricultural areas. Dust from the petrol combustion, brake linings, and from wearing of tires are potential sources of Cd (Oliver 1997; Batayneh et al. 2015; Lin et al. 2005; Radziemska and Fronczyk 2015). Cd content in soil samples exceeded the critical concentration of Cd (of about 3 to 8 mg/kg) (Raven et al. 1998; Adriano 2001). In addition, Cd is widely distributed in the earth’s crust at an average content of about 0.1 mg/kg. Jarrar and Mustafa (1995) recorded high levels of Cd ranging from 7 to 34 mg/kg in the bituminous limestone of Irbid region, Jordan. Mustafa et al. (1998) reported an average Cd in the northern Jordanian phosphates of 33 mg/kg.
Zinc (Zn)
Zn content ranged between 22.0 and 77.0 mg/kg with an average 45.5 mg/kg (Tables 1 and 3). On a global basis, the average Zn concentration in soils is 64 mg/kg, whereas the mean Zn for Cambisols is 60 mg/kg (Table 3). Zn exhibited medium levels in roadside soils, with relatively higher concentrations in sampling sites 1 (77 mg/kg) and 2 (75 mg/kg). Wear and corrosion of vehicle parts and oil floods from auto-repair workshop close to these sites are probable sources of Zn. Zn is used as an antioxidant in the engine oil (Adachi and Tainosho 2004; Contini et al. 2012). The concentrations of Zn found in the present study are lower than that of roadside soil of Irbid/Zarqa Highway, Jordan reported by Odat (2013a, b) (Table 4 and Fig. 7).
Chromium (Cr)
Cr level ranged between 12 and 22 mg/kg with an average of 15.9 mg/kg, which is lower than the world average Cr content of about 70 mg/kg (Table 3). Relatively higher concentrations of Cr were observed at site 1 and site 2 of 22 and 20 mg/kg, respectively. The low values of Cr content in soil samples (compared to world soil average content of Cr) are suggestive of geogenic origin, particularly from chemical weathering of basalt rocks exposed in the Wadi Al-Arab catchment (Figs. 1 and 2). Mielke (1979) measured Cr content of about 170 mg/kg in basaltic rocks and 11 mg/kg in limestone, whereas the average crustal abundance of 122 mg/kg was detected by Kabata-Pendias and Pendias (2001).
Copper (Cu)
Cu content in soil samples varied from 2.0 to 8.7 mg/kg with an average of 4.1 mg/kg (Tables 1 and 3). Kabata-Pendias and Pendias (2001) reported average Cu levels in soil varying from 13 to 24 mg/kg. Similarly, low Cu content in the studied topsoil, is indicative of natural sources, though minor contribution from anthropogenic sources is likely (fungicide and pesticides enriched in Cu (Jones and Jarvis 1981)). The lower levels of Cu in soil samples can be attributed to synthesize of coordinated chelate complex compounds with humic acids of soils and can substitute Al3+ at octahedral sites when interacting with clay minerals in these soils (Pinskii et al., 2018). In addition to that, the adsorption of metals by soils and the strength of their fixation on the surface of soil particles under both mono- and poly-element contamination decreased with the decreasing proportion of fine fractions in the soil (Minkina et al. 2011).
Nickel (Ni)
Ni concentration in soil samples ranged from 33.0 to 88.0 mg/kg with an average of 59.8 mg/kg. While these values are lower than the critical Ni concentration in soil (of around 100 mg/kg (ref?)), they are greater than the world average concentrations of Ni in soil (of 40 mg/kg (ref?)). Slightly higher levels of Ni were observed in sampling sites 1, 2, and 10 which might be ascribed to the fact that Ni is fuel additives, especially in burning (diesel) used in fuel engines and residential heating systems (Loranger and Zayed 1994). In addition to partial release from rocks (oil shale and bituminous sediments), Ni is emitted from motor vehicle exhaust and car repair shops. Ni content is positively correlated with Cr, Co, Cd, Cu, Pb, and Zn in soils (Table 2).
Environmental pollution indices
Based on Muller scales (1981), the soil samples are uncontaminated with Ni, Zn, Co, and Cu (Igeo (1) = − 0.396, − 0.4, − 0.137, and − 1.397, respectively), uncontaminated to moderately contaminated with Cr, and Pb (Igeo (1) = 0.025, and 0.56, respectively) and moderately contaminated with Cd (Igeo (1) = 1.65). On the basis of mean Igeo (1) values calculated according to the average crustal abundance as background value (Fortescue 1992), heavy metals are ranked in the following order: Pb (56.5) > Cd (1.65) > Cr (0.025) > Ni (− 0.396) > Zn (− 0.4) > Co (− 0.137) > Cu (− 1.397) (Table 3 and Fig. 8).
The average Igeo (2) values, calculated based on the average GGBC of Kabata-Pendias (2011), soils are uncontaminated with Zn, Cr, Co, Cu (Igeo (2) = − 0.12, − 0.68, − 0.70, and − 0.91, respectively), and uncontaminated to moderately contaminated with Pb and Ni (Igeo (2) = 0.28, and 0.185) respectively, moderately contaminated with Cd (Igeo (2) = 1.2). The heavy metals are ranked in the following order (based on Igeo (2)): Cd (1.2) > Pb (0.28) > Ni (0.185) > Zn (− 0.12) > Cr (− 0.68) > Co (− 0.7) > Cu (− 0.91) (Table 3 and Fig. 8).
The average Igeo (3) values calculated based on the average geochemical background level in control samples (MGBLC) from Koforyooba sampling sit (Fig. 1), soils are uncontaminated with Cu, Co, Cr, and Ni (Igeo (3) = − 1.90, − 0.21, − 0.11, and − 0.05, respectively), and uncontaminated to moderately contaminated with Zn, Pb, and Cd (Igeo (3) = 0.03, 0.26, and 0.86) respectively. The heavy metals are ranked in the following order (based on Igeo (3)): Cd (0.86) > Pb (0.26) > Zn (0.03) > Ni (− 0.05) > Cr (− 0.11) > Co (− 0.21) > Cu (− 1.9) (Table 3 and Fig. 8).
Different contamination indices have been calculated and presented in Figs. 9 and 10. The contamination factors (CFs) for the selected heavy metals in different sites are ranging from 0.1 (Cu) to 44.4 (Cd), with the following increasing order: Cu < Cr < Zn < Ni < Pb < Co < Cd. The CF values decreased in soil samples from sites 3, 4, 5, 6, 7, and 8. The high values of the average CFs (> 1) in Cd (23.8), Co (3.2), Pb (2.6), and Ni (1.9) indicated that soils are very highly contaminated with Cd, considerably contaminated with Co, and moderately contaminated with Pb and Ni (Hakanson 1980). The overall average of Cr, Co, Cd, Cu, Pb, Zn, and Ni contamination in the studied soil samples followed the decreasing order: site 1 = 2 > site 3 = 4 > site 19 = 20 > site 17 = 18 > site 15 = 16 > site 13 = 14. Sampling sites 1,2, 3, 4, 19, and 20 are in close proximity to commercial or industrial areas as Irbid industrial city, whereas sites 5, 6, 7, 8, 9, 10, 11, and 12 are located nearby residential or agricultural areas.
The results showed that the sampled soils are less contaminated with Cr, Cu, and Zn, with CF average values of 0.32, 0.21, and 0.76, respectively (< 1 CF class) (Fig. 9). In addition, the PLI values for Cr (0. 2), Cu (0.1), Zn (0.6) are regarded as not polluted. However, the average PLI values of soil samples were greater than one (> 1) for Cd (13.3), Co (5.2), and Pb (3.6) and Ni (2.4) (Figs. 9 and 10) indicating a probable environmental pollution by these metals (from agrochemicals, oil leakage, vehicle spare parts, etc).
The calculated Ei indicated that the majority of heavy metals pose a low risk (Ei < 40) to soil, except for Cd, which showed an Ei value of 399 and is considered a significantly high risk (Hakanson 1980) (Fig. 10). Heavy metals pose considerable potential ecological risk with PERI value of 492 (Hakanson 1980). Statistically, more than 75% of the estimated ecological risk is related to Cd (Figs. 9 and 10), which revealed that serious Cd pollution in most sampling sites along the highway.
Clay fraction, organic matters, and CaCO3 played an important role in bounding anthropogenic Cr, Co, Cd, Cu, Pb, Ni, and Zn to the soil particles (El-Radaideh 2010; Al-Taani et al. 2015a; El-Radaideh et al. 2017a). Heavy metals in soils are of geogenic and anthropogenic origins. Weathering of rocks in the Wadi Al-Arab catchment is the primary natural source, whereas agrochemicals, vehicle exhausts, degradation of surface wear and paint of vehicles, vehicle wear debris of tire, brake lining are the main anthropogenic sources of heavy metals.
Conclusion
This study provides valuable results about heavy metal contents in soils along Irbid-North Shooneh highway. The concentrations of heavy metals in soil samples were as follows: Pb > Ni > Zn > Co > Cr > Cd > Cu. These soils contain high Cd, Co, Pb, and Ni concentrations in comparison to background Cambisols, whereas Cr, Zn, and Cu concentrations were below their world average content in soil. The correlation analysis of average concentrations showed positive correlations among heavy metals, suggesting that these elements have common sources. The results of Igeo, CF, Ei, and PERI indicated that Cd is the main soil pollutant. The observed levels of heavy metals are attributed to vehicular traffic and the agricultural activities. This assessment can serve as a baseline data for future studies on top and subsoil of Irbid-North Shooneh area, with particular emphasis on the potential impacts of heavy metals on plants.
References
Abu-Rukah Y, Samawi M (2000) A study of contamination of the northern Jordan valley agricultural fields by various metal ions using regression analysis. Qatar Univ J 20:155–165
Abu-Rukah, Y.H., (2002). A study of soils along the Amman Aqaba highway contamination with heavy metals. Freiberger Forschungshefte, Geowissenschaften C494:53–62
Adachi K, Tainosho O (2004) Characterization of heavy metal particles embedded in tire dust. Environ Inter 30(8):1009–1017
Adamiec E, Jarosz-Krzemińska E, Wieszała R (2016) Heavy metals from non-exhaust vehicle emissions in urban and motorway road dusts. Environ Monit Assess 188:369
Adriano D (2001) Trace elements in terrestrial environments: biogeochemistry, bioavailability, and risks of metals. Springer, New York
Al-Khashman O, Shawabkeh R (2009) Metal distribution in urban soil around steel industry beside queen Alia airport, Jordan. Environ Geochem Hlth 31(6):717–726
Al–Khashman OA, Shawabkeh RA (2006) Metals distribution in soils around the cement factory in southern Jordan. Environ Pollut 140:387–394
Allen SE, Grimshaw HM, Parkinson JA, Quarmby C (1974) Chemical analysis of ecological materials. Blackwell, Oxford
Al-Qudah B (2001) Soils of Jordan. In: Zdruli, P. Steduto, C. Lacirignola, L. Montanarella (eds) Soil resources of southern an eastern Mediterranean countries. Options Méditerranéennes, Série B
Alsbou E, Al-Khashman O (2018) Heavy metal concentrations in roadside soil and street dust from Petra region, Jordan. Environ Monit Assess 190(48). https://doi.org/10.1007/s10661-017-6409-1
Al-Shereideh S, Wahsha M, El-Radaideh N, Al-Taani A, Abderahman N, Odat A, Al-Momani T, Khawajah M (2015) Geo-environmental Assessment of Al-Ramtha Soils, Jordan. Curr Wor Environ 10(2):386–397
Al-Taani AA, Batayneh A, El-Radaideh N, Al-Momani I, Rawabdeh A (2012) Monitoring of selenium concentrations in major springs of Yarmouk Basin, North Jordan. World App Scs J 18(5):704–714
Al-Taani AA, Batayneh A, Mogren S, Nazzal N, Ghrefat H, Zaman H, Elawadi E (2013) Groundwater quality of coastal aquifer systems in the eastern coast of the Gulf of Aqaba, Saudi Arabia. J Appl Sci Agric 8(6):768–778
Al-Taani AA, Batayneh A, El-Radaideh N, Ghrefat H, Zumlot T, Al-Rawabdeh A, Al-Momani T, Taani A (2015a) Spatial distribution and pollution assessment of heavy metals in surface sediments of Ziqlab reservoir, Jordan. Environ Monit Assess 187(32):1–14
Al-Taani AA, Rashdan M, Khashashneh S (2015b) Atmospheric dry deposition of mineral dust to the Gulf of Aqaba, Red Sea: rate and trace elements. Marine Pollut Bull 92(1–2):252–258
Angulo E (1996) The Tomlinson pollution load index applied to heavy metal “mussel–watch” data: a useful index to assess coastal pollution. Sci Total Environ 187(1):19–56
Apeagyei E, Bank MS, Spengler JD (2011) Distribution of heavy metals in road dust along an urban-rural gradient in Massachusetts. Atmos Environ 45(13):2310–2323
ATSDR (Agency for Toxic Substances and Disease Registry) (2007) Toxicological profile for chromium
Babula P, Adam V, Opatrilova R, Zehnalek J, Havel L, Kizek R (2008) Uncommon heavy metals, metalloids and their plant toxicity: a review. Environ Chem Lett 6:189–213
Banat KM, Howari FM, Al–Hamad AA (2005) Heavy metals in urban soils of central Jordan: should we worry about their environmental risks? Environ Res 97:258–273
Batayneh A, Al-Taani AA (2015) Integrated resistivity and water chemistry for evaluation of groundwater quality of the Gulf of Aqaba coastal area in Saudi Arabia. Geosci J 20(3):403–413
Batayneh A, Elawadi E, Zaman H, Al-Taani AA, Nazzal Y, Ghrefat H (2014) Environmental assessment of the Gulf of Aqaba coastal surface waters, Saudi Arabia. J Coast Res 30(2):283–290
Batayneh A, Ghrefat H, Zumlot T, Elawadi E, Mogren S, Zaman Z, Al-Taani AA, Nazzal Y, Elwaheidi M (2015) Assessing of metals and metalloids in surface sediments along the Gulf of Aqaba coast, northwestern Saudi Arabia. J Coast Res 31(1):163–176
Bender F (1968) Geologie von Jordanien, Beiträge zur Regionalen Geologie der Erde, Band 7.- Gebrüder Bornträger; Berlin
Bretzel F, Benvenuti S, Pistelli L (2014) Metal contamination in urban street sediment in Pisa (Italy) can affect the production of antioxidant metabolites in Taraxacum officinale Weber. Environ Sci Pollut Res Int 21(3):2325–2333
Carroll D (1970) Clay minerals: a guide to their X-ray identification. Geological Society of America. Special Paper No. 126. pp 80
Charlesworth SM, Everett M, McCarthy R, Ordonez A, de Miguel E (2003) A comparative study of heavy metal concentration and distribution in deposited street dusts large and a small urban area: Birmingham and Coventry, west midlands, UK. Environ Inter 29(5):563–573
Chen TB, Zheng YM, Lei M, Huang ZC, Wu HT, Chen H, Fan KK, Yu K, Wu X, Kocher B, Wessolek G, Stoffregen H (2005) Water and heavy metal transport in roadside soils. Pedosphere 15(6):746–753
Christoforidis A, Stamatis N (2009) Heavy metal contamination in street dust and roadside soil along the major national road in Kavala’s region, Greece. Geoderma 151(3–4):257–263
Contini D, Belosi F, Gambaro A, Cesari D, Stortini AM, Bove MC (2012) Comparison of PM10 concentrations and metal content in three different sites of the Venice Lagoon: an analysis of possible aerosol sources. J Environ Scis 24:954–1965
Elliot HA, Liberati MR, Huang CP (1986) Competitive adsorption of heavy metals by soils. J Environ Qual 15:214–219
Ellis JB, Revitt DM (1982) Incidence of heavy metals in street surface sediments: solubility and grain size studies. Water Air Soil Pollut 17:87–100
El-Radaideh N (2010) Using bottom reservoir sediments as a source of agricultural soil: Wadi El-Arab reservoir as a case study, NW Jordan. Abhath Al-Yarmouk 19(2):75–91
El-Radaideh N (2016) Geoenvironmental assessment of Al-Wala reservoir sediments. Jordan Geosci J 20:77–87. https://doi.org/10.1007/s12303-0150022-5
El-Radaideh N, Al-Taani AA, Al-Momani T, Tarawneh K, Batayneh A, Taani A (2014) Evaluating the potential of sediments in Ziqlab reservoir (Northwest Jordan) for soil replacement and amendment. Lake Reser Manag 30:32–45
El-Radaideh N, Al-Taani AA, Al Khateeb W (2017a) Status of sedimentation in King Talal Dam, case study from Jordan. Environ Earth Scs 76(3):132
El-Radaideh N, Al-Taani AA, Al Khateeb W (2017b) Characteristics and quality of reservoir sediments, Mujib dam, Central Jordan, as a case study. Environ Monit Assess 189(4):143. https://doi.org/10.1007/s10661-017-5836-3
Faiz Y, Tufail M, Javed MT, Chaudhry MM, Siddique N (2009) Road dust pollution of Cd, Cu, Ni, Pb and Zn along Islamabad expressway, Pakistan. Microchem J92:186–192
Fergusson JE, Hayes RW, Yong TS, Thiew SH (1980) Heavy metal pollution by traffic in Christchurch. New Zealand: lead and cadmium content of dust and soil, and plant samples. N Z J Sci 23:293–310
Ferreira-Baptista L, De Miguel E (2005) Geochemistry and risk assessment of street dust in Luanda, Angola: a tropical urban environment. Atmosph Environ 39:4501–4512
Folk RL (1974) Pertology of sedimentary rocks. Hemphill Publishing, Austin (TX), p 182
Fortescue J (1992) Landscape geochemistry-Retrospect and prospect-1990. Appl Geoch 7:1–54
Grim RE (1968) Clay mineralogy, 2nd edn. McGraw-Hill, New York (NY), p 596
Gee GW, Bauder JW, (1986) Particle size analysis. In Klute A (ed) Methods of soil analysis Part 1. Physical and mineralogical methods. Agronomy series No. 9. American Society of Agronomy, Madison
Hakanson L (1980) An ecological risk index for aquatic pollution control sedimentological approaches. Wat Resear 14(8):975–1001
Hesse PR (1972) Textbook of soil chemical analysis. Chemical Publications, New York (NY), p 520
Hewitt CN, Candy GBB (1990) Soil and street dust heavy metal concentrations in and around Cuenca, Ecuador. Environ Pollut 63(2):129–136
Howari FM, Banat KM (2001) Assessment of Fe, Zn, Cd, Hg, and Pb in the Jordan and Yarmouk River sediments in relation to their physicochemical properties and sequential extraction characterization. Water Air Soil Pollut 132(1–2):43–59
Howari FM, Abu-Rukah Y, Goodell PC (2004) Heavy metal pollution of soils along north Shooneh-Aqaba highway, Jordan. Internt J Environ Poll 22:597. https://doi.org/10.1504/IJEP.2004.005913
Ibrahim M, Al-Mashakbeh H (2016) Integrating lithostratigraphic units and GIS-analysis techniques to modified surface water quality index. J Environ Prot 07:1104–1112. https://doi.org/10.4236/jep.2016.78099
IUSS Working Group WRB (2007) World reference base for soil resources 2006, first update 2007. World soil resources reports no. 103. FAO, Rome
Jarrar M, Mustafa H (1995) Mineralogical and geochemical study of the oil shale of Wadi Esh-Shallalah (NW Jordan). Abhath Al-Yarmouk 4:111–136
Jones L H P, Jarvis S C (1981) The fate of heavy metals,” in The Chemistry of Soil Processes, D. J. Green and M. H. B. Hayes, Eds., p. 593, John Wiley and Sons, New York, NY, USA
Kabata-Pendias A (2011) Trace elements in soils and plants, 4th edn. CRC Press, Boca Raton. https://doi.org/10.1201/b10158-25
Kabata-Pendias A, Pendias H (2001). Trace elements in soils and plants, CRC Press, Boca Raton, Fla, USA
Kelly J, Thornton I, Simpson PR (1996) Urban geochemistry: a study of the influence of anthropogenic activity on heavy metal content of soils in traditionally industrial and non industrial areas of Bristol. Appl Geochem 11:363–370
Khresat SA, Rawajfih Z, Mohammad M (1998) Land degradation in north-western Jordan: causes and processes. J Arid Environ 39:623–629
Kim JA, Park JH, Hwang WJ (2016) Heavy metal distribution in street dust from traditional markets and the human health implications. Int J Environ Res Public Health 13:820
Kluge B, Wessolek G (2012) Heavy metal pattern and solute concentration in soils along the oldest highway of the world—the AVUS autobahn. Environ Monit Assess 184:6469–6481
Kraushaar S (2016) Soil Erosion and sediment flux in northern Jordan, Springer Theses, 185p. https://doi.org/10.1007/978-3-319-31888-2_2
Li L (2005) Retention capacity and environmental mobility of Pb in soils along highway corridor. WATER AIR SOIL POLL170:211–227
Lin CC, Chen SJ, Huang KL, Hwang WI, Chang-Chien GP, Lin W (2005) Characteristics of metals in nano/ultrafine/fine/coarse particles collected beside a heavily trafficked road. Environ Sci Tech 39:8113–8122
Liu E, Yan T, Birch G, Zhu Y (2014) Pollution and health risk of potentially toxic metals in urban road dust in Nanjing, a mega-city of China. Sci Total Environ 476-477:522–531
Loranger, Zayed J (1994) Manganese and lead concentrations in ambient air and emission rates from unleaded and leaded gasoline between 1981 and 1992 in Canada: a comparative study. Atmos Environ 1994 28(9):1645–1651
Loring D, Rantala R (1992) Manual for the geochemical analyses of marine sediments and suspended particulate matter. Earth-Sci Reviw 32:235–283
Loska K, Wiechuła D, Barska B, Cebula E, Chojnecka A (2003) Assessment of arsenic enrichment of cultivated soils in southern Poland. POL J Environ Stud12:187–192
Madrid L, Barrientos ED, Madrid F (2002) Distribution of heavy metal contents of urban soils in parks of Seville. Chemosphere 49:1301–1308. https://doi.org/10.1016/S0045-6535(02)00530-1
Manta DS, Angelone M, Bellanca A, Neri R, Sprovieri M (2002) Heavy metals in urban soils: a case study from the city of Palermo (Sicily), Italy. Sci Total Environ 300:229–243. https://doi.org/10.1016/S0048-9697(02)00273-5
Mashal K, Al-Qinna M, Ali Y (2009) Spatial distribution and environmental implications of lead and zinc in urban soils and street dusts samples in Al-Hashimeyeh municipality. Jordan J Mech Ind Eng 3(2):141–150
Meyer WL, Marsh M, Fup PA (1994) Cation exchange capacities of upland soils in eastern Canada. Canad J Soil Sci 74:393–408
Mielke JE (1979) Composition of the Earth’s crust and distribution of the elements. In: Siegel FR (ed) Review of research on modern problems in geochemistry. UNESCO Report, Paris, pp 13–37
Minkina TM, Mandzhieva SS, Sushkova SN, Pinskii DL, Antonenko EM (2011) Effect of the particle-size distribution on the adsorption of copper, lead, and zinc by Chernozemic soils of Rostov oblast. Eurasian Soil Sci 5(44):1193–1200
Mmolawa K, Likuku A, Gaboutloeloe G (2011) Assessment of heavy metal pollution in soils along roadside areas in Botswana. Afr J Environ Sc Tech 5(3):186–196
Moreno T, Karanasiou A, Amato F, Lucarelli F, Nava S, Calzolai G, Chiari M, Coz E, Artíñano B, Lumbreras J, Borge R, Boldo E, Linares C, Alastuey A, Querol X, Gibbons W (2013) Daily and hourly sourcing of metallic and mineral dust in urban air contaminated by traffic and coal-burning emissions. Atmos Environ 68:33–44
Muller G (1981) The heavy metal pollution of the sediments of Neckars and its tributary. A Stock taking Chemische Zeit 150:157–164
Mustafa H, Attalla M, Khoury H (1998) The upper cretaceous phosphate in NW-Jordan. Abhath Al-Yarmouk 7(1):73–114
Nazzal Y, Rosen MA, Al-Rawabdeh AM (2013) Assessment of metals pollution in urban road dust from selected highways of the greater Toronto area in Canada. Environ Monit Assess 185(2):1847–1858
Odat S (2013a) Calculating pollution indices of heavy metal along Irbid/Zarqa highway-Jordan. Int J Appl Sci Tech 3(8):72–76
Odat S (2013b) Calculating pollution indices of heavy metal along Irbid/Zarqa highway-Jordan. Int J Appl Sci Tech 3(8):72–76
Oliver MA (1997) Soil and human health, a review. Eur J Soil Sci 48(4):573–592
Pinskii DL, Minkina TM, Bauer TV, Nevidomskaya DG, Mandzhieva SS, Burachevskaya MV (2018) Copper adsorption by chernozem soils and parent rocks in southern Russia. Geochem Int 56(3):266–275. https://doi.org/10.1134/S0016702918030072
Rachwal M, Magiera T, Wawer M (2014) Coke industry and steel metallurgy as the source of soil contamination by technogenic magnetic particles, heavy metals and polycyclic aromatic hydrocarbons. Chemosphere 138:863–873
Radziemska M, Fronczyk J (2015) Level and contamination assessment of soil along an expressway in an ecologically valuable area in Central Poland. Int J Environ Res Public Health 12:13372–13387
Raven P H, Berg L R, Johnson G B (1998) Environment, Saunders College Publishing, New York, NY, USA, 2nd edition
Salmons W, Forstner U (1984) Metals in the hydrocycle. Springer Verlag, Berlin
Sharma S, Prasad FM (2010) Accumulation of lead and cadmium in soil and vegetable crops along major highways in Agra (India). Environ-J Chem 7:1174–1183
Tian QZ (2005) Assessment of heavy metal pollution in surface soils of urban parks in Beijing, China. Chemosphere 60:542–551
Tomllinson DC, Wilson JG, Harris CR, Jeffrey DW (1980) Problems in the assessment of heavy metals levels in estuaries and the formation of pollution index. Helgoländer Meeresun 33:566–569
Turer D, Maynard JB, Sansalone JJ (2001) Heavy metal contamination in soils of urban highways: comparison between runoff and soil concentrations at Cincinnati, Ohio. Water, AIR Soil Pollut 132:293–314
Ubwa ST, Abah J, Ada CA, Alechenu E (2013) Levels of some heavy metals contamination of street dust in the industrial and high traffic density areas of Jos Metropolis. J Biodivers Environ Sci 3(7):13–21
USEPA,(United States Environmental Protection Agency) (2002) Risk assessment: technical background information. http://www.epa.gov/reg3hwmd/risk
Wang G, Hou-Qi L, Gong Y, Wei Y, Ai-Jun M, Liu-Yan Y, Zhong H (2017) Risk assessment of metals in urban soils from a typical Industrial City, Suzhou, eastern China. Int J Environ Res Public Health 14(9):1025
Wei B, Yang L (2010) A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem J 94(2):99–107
Williams TM, Rees JG, Setiapermana D (2000) Metals and trace organic compounds in sediments and waters of Jakarta Bay and the Pulau Seribu Complex, Indonesia. Mar Pollut Bull 40:277–285
Yusuf N, Al-Taani AA, Al-Fukaha F, Al-Shereideh S, Mahasneh M (2011) Sediments transport across the fringing reef in the Gulf of Aqaba, Red Sea and the implications on live corals. Abhath Al-Yarmouk: "Basic Sci& Eng" 20(1):35–52
Zhang H, Zhang Y, Wang Z, Ding M (2013) Heavy metal enrichment in the soil along the Delhi-Ulan section of the Qinghai-Tibet railway in China. Environ Monit Assess 185:5435–5447
Acknowledgments
The authors gratefully acknowledge use of the services and facilities at Yarmouk University and Al Al-Bayt University, Jordan, and the data provided by Jordan Agricultural Ministry.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Radaideh, N.M., Al-Taani, A.A.AK. Geo-environmental study of heavy metals of the agricultural highway soils, NW Jordan. Arab J Geosci 11, 787 (2018). https://doi.org/10.1007/s12517-018-4099-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-018-4099-9