Abstract
A series of silica sorbents with different content of amidoxime groups were prepared through co-condensation method and applied to extract uranium from saline lake brine. The optimum amidoxime group content was determined and effects of pH on uranium sorption were investigated. Sorption kinetic and isotherms were also investigated. XPS analysis indicated that the adsorption mechanism of uranium was attributed to the interaction between uranyl ion and N in the amidoxime. Amidoximated silica could efficiently absorb the naturally occurring uranium in the saline lake brine samples from Qinghai, China.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human life has relied upon fossil fuels since the beginning of the industrial era. Limited fossil resources and environmental constraints have aroused interesting in alternative energy sources [1]. Nuclear power can meet the growing energy demand caused by fossil fuel scarcity [2]. However, sharp increase in nuclear power will put a heavy burden on uranium reserves and uranium ore will be insufficient in the further. Uranium in seawater is 103 larger than the terrestrial resources [3, 4]. Unfortunately, uranium extraction from seawater is difficult for the extremely low uranium concentration (2.8–3.3 μg/L). The uranium concentrations in some saline lakes were much higher than that in seawater and the advantage in uranium concentration makes saline lake brine an ideal resource for uranium [5, 6].
Sorption is an effective method for uranium recovery from aqueous for the benefits of simplicity, rapidity and easy recycling [7]. To make uranium extraction feasible, solid adsorbents which are effective under saline lake brine conditions should be developed. Mesoporous silica is a kind of excellent adsorbent owing to its high specific surface area and uniform pore-size distribution [8, 9]. Vidya et al. [10, 11] found that the adsorption of \({\text{UO}}_{ 2}^{{ 2 { + }}}\) onto MCM-41 and MCM-48 attributed to direct template-ion exchange. However, pure silica adsorbents could hardly adsorb uranium from saline lake brine since the limitation in selectivity. Organic functionalized silica have been synthesized in order to obtain specific active sites on the silica surface [12]. Amidoxime group containing nucleophilic NH2 and hydroxyl functionality, which can effectively binding uranium ion, is a promising chelating function groups for uranium adsorption from aqueous phase [13,14,15,16,17]. Amidoximated mesoporous silica is an effective adsorbent for uranium sorption from aqueous solutions and simulated seawater, but its sorption property for uranium from natural seawater and saline lake brine is indefinite [18, 19]. The concentration of Li+, K+, Na+, Mg2+ were 102–105 times more than the concentration of uranium in the natural seawater and saline lake brine, so selectivity is the crucial factor for the extraction of uranium from these natural water. Selectivity of amidoximated mesoporous silica for uranium over other inorganic ions in saline lake brine is unknown.
In present work, MCM-41 type silica adsorbents with different content of amidoximated groups were prepared via co-condensation method which was more concise and efficient than post-synthesis grafting method. FTIR and XPS analysis were carried out to characterize the prepared adsorbents. The optimum amidoxime group density was determined and effects of pH on uranium sorption were investigated. Sorption kinetic and isotherms were also investigated. Mechanism of uranium adsorption was conjectured base on the adsorption experiments and XPS analysis. The performance of prepared adsorbents in uranium adsorption from natural saline lake brine was evaluated.
Experimental
Materials
2-Cyanoethyltriethoxysilane (CTES) was purchased from Sigma-Aldrich (Shanghai) Trading Co., Ltd. Saline lake brine samples were collected from different salt pan in Qinghai Province, China. All reagents were of analytical reagent grade and used without purification.
Preparation of amidoximated silica
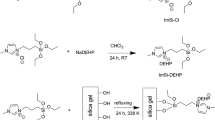
Amidoximated silica adsorbents were prepared according to the following steps: 1 g of CTAB was dissolved in 135 ml of deionized water and then 102 mL of ammonia was added to the solution. After stirring for 0.5 h, CTES and 5 mL of TEOS were mixed and added to the homogeneous solution. The mixture was stirred for 2 h at room temperature. The powder were recovered by filtration, purified with plenty of 10/90 HCl/C2H5OH and dried at 60 °C. The specific molar ratio of CTES/TEOS was obtained by controlling the quantity of CTES. The obtain silica grafted with nitrile(–CN) named CMCM-X (X = 0, 0.2, 0.4 or 0.6) were treated with 3 g NH2OH·HCl in 100 mL 50/50 H2O/CH3OH solution for 72 h at 80 °C. Sodium carbonate was used to adjust the solution pH to neutral. The final products, denoted as AMCM-X, were washed with deionized water and dried at 60 °C.
Characterization
FTIR were carried out in a Bruker Tensor 27 at room temperature. XPS of samples were collected on an ESCALAB 250Xi (Thermo Fisher Scientific).
Sorption experiments
Batch adsorption experiments were performed to investigate the process of uranium adsorption from aqueous solution on AMCM-X. 5 mg of adsorbents were added into polyethylene tubes containing 5 mL of uranium solution. The solution pH was adjusted by adding negligible volumes of HNO3 or NaOH solution. The tubes were shaken at 300 rpm and then the uranium loaded sorbents (AMCM-X-U) were collected. Uranium concentrations in the supernatants were determined by UV–Vis spectrophotometer. The uranium sorption capacity (q e, mg g−1) was calculated according to the Eq. (1):
where C 0 is the initial uranium concentration in solution (mg L−1), C e is the equilibrium uranium concentration in solution (mg L−1), V is the volume of uranium solution (mL) and m is the weight of adsorbent (mg), respectively.
Desorption experiments
Desorption of uranium from uranium-loaded amidoximated silica were conducted at room temperature by 5 mL HCl, Na2CO3 and NaOH solution. The uranium concentrations in desorbing solutions were determined after shaking for 2 h. The desorption ratio (%) was calculated by Eq. (2):
Uranium adsorption from saline lake brine samples
Uranium adsorption from saline lake brine samples with AMCM-0.4 were conducted at room temperature. 5 mg of AMCM-0.4 was contacted with 50 mL of saline lake brine samples in flasks. After stirring with a magneton for 7 days, brine samples were purified by centrifugation. Uranium concentrations in brine samples before and after adsorption were measured by pulsed-ultraviolet induced fluorescence method [20]. The uranium sorption capacity (q se, mg g−1) from saline lake brine was calculated according to Eq. (3):
where C si and C sf are the initial and final uranium concentrations in brine samples (mg L−1), V is the volume of saline lake brine (mL).
Selectivity coefficients were calculated by Eqs. (4) and (5)
where A 1 is the amount of inorganic ion in aqueous solution at equilibrium, A 2 is the amount of inorganic ion on adsorbent at equilibrium. [21] Concentrations of Li, Na, K and Mg in saline lake brine were detected by ICP-AES.
Results and discussion
Characterization
Figure 1 showed the FTIR spectra of CMCM-0, CMCM-0.4, AMCM-0.4 and AMCM-0.6. The large broad band at 3427 cm−1 and the band at 1630 cm−1 were attributed to the stretching vibration of Si–OH group and the adsorbed water [7, 22]. The bands at 1085, 800 and 470 cm−1 were related to the stretching vibration, typical symmetric and bending vibrations Si–O–Si in the silica, respectively. The stretching band of the free silanol groups at 960 cm−1 was also observed [23]. Furthermore, the undesirable faint peaks at 2927 and 2856 cm−1, which were attributed to the characteristic asymmetric and symmetric vibration of CH2 bands, demonstrated the trace residue of CTAB on CMCM-0 [24]. In comparison with the spectrum of CMCM-0, the characteristic stretching vibration absorption band of cyano groups at 2253 cm−1 was observed in the spectrum of CMCM-0.4. After amidoximation, the band of cyano groups extremely weakened. The vibration absorption of C=N (expected at 1660 cm−1) and N–OH (expected at 944 cm−1) in the amidoxime group on AMCM-0.4 couldn’t be confirmed directly by FTIR spectrum due to overlap of the strong O–H band and the free silanol groups, respectively. In fact, the broad peak at 1651 cm−1 in the spectrum of AMCM-0.4 was the combination of the peaks of O–H and C=N groups. These confirm that cyano groups were converted to amidoxime groups by treatment with hydroxylamine [19]. The absorption band at 2253 cm−1 in the spectrum of AMCM-0.6 illustrate that only a portion of the cyano groups on CMCM-0.6 were converted to amidoxime groups in spite of sufficient of hydroxylamine and adequate reaction time. CTES could not disperse sufficiently in TEOS during preparation of CMCM-0.6 and the cyano groups on CMCM-0.6 were clustered resulted in the partial amidoximation of the cyano groups. The above information confirmed the successful synthesis of amidoximated silica.
XPS analysis was used to investigate the surface chemical composition and bonding properties of CMCM/AO-X. Figure 2 showed the survey spectra of AMCM-X. For AMCM-0, the main elements were Si and O, and the C 1s peak was attributed to the trace residue of CTAB. N was detected in the spectra of CMCM-0.4 and AMCM-0.4. The peak at about 399.6 eV in N 1s core-level spectrum as shown in Fig. 3 indicated the existence of –CN [25]. Furthermore, the two peak components at 102.7 and 103.5 eV in the Si 2p core-level spectrum of CMCM-0.4 were attributed to the (–O)3SiCH2 and SiO2 species, respectively [26, 27]. Existence of –CN and (–O)3SiCH2 indicated that CTES was incorporated into CMCM-0.4 successfully. After amidoximation, obvious position change of N 1s core-level spectrum was observed. The two fitted peaks at binding energies of 399.4 and 400.4 eV were attributed to the nitrogen atoms in –NH2 and C=N–OH species, respectively [28, 29]. N 1s core-level spectra demonstrated the successful amidoximation of cyano.
Effect of amidoxime group content on uranium sorption
Figure 4 showed that the uranium sorption capacity on AMCM-X increased as X increase from 0 to 0.6. Obviously, amidoxime group on the AMCM-X was beneficial to adsorption of uranium. However, insufficient amidoximation of CMCM-0.6 resulted in uranium adsorption capacities on AMCM-0.4 and AMCM-0.6 were comparable. Accordingly, AMCM-0.4 was selected for the further experiments.
Effect of initial pH on uranium sorption
In order to avoid the precipitation of uranium, initial solution pH value was limited within 4.5 [30]. As shown in Fig. 5, in the pH range of 1–4.5, the uranium(VI) sorption onto amidoximed silica increased with increasing pH. Maximum uranium sorption capacity was obtained at pH 4.5. Such a pH-dependent sorption was attributed to the surface charge of adsorbent and the uranium speciation. When pH < 3, uranium in solution was in the form of \({\text{UO}}_{2}^{2 + }\) and the amidoxime groups were protonated. The electrostatic repulsion between AMCM-X and \({\text{UO}}_{2}^{2 + }\) resulted in that scarce uranium was absorbed by AMCM-X when pH < 3. As the pH increased, protonated amidoxime gradually become electrically neutral and \({\text{UO}}_{2}^{2 + }\) ion gradually hydrolyze to UO2(OH)+. Therefore, the uranium adsorption capacity onto AMCM-X increased with increase of pH. Uranium was also absorbed at pH 4.5 by AMCM-0, the silica adsorbent with no amidoxime, indicated that uranium removal by AMCM-X was the co-effect of both silica and amidoxime group.
Effect of time on uranium sorption and sorption kinetics
Effect of time on uranium sorption by AMCM-0.4 was depicted in the form of q t versus contact time, and the sorption kinetic was discussed with non-linear pseudo-second-order kinetic model expressed as Eq. (6) [31]:
where q t (mg g−1) is the amount of uranium adsorbed at contact time t (min), q e is the equilibrium uranium sorption capacity (mg g−1), and k 2 is the pseudo -second-order rate constant (g mg−1 min−1) [20].
As shown in Fig. 6, the uranium adsorption capacity on AMCM-0.4 increased quickly in the initial 30 min. The fast sorption kinetic could be attributed to the combination of uranium and plentiful amidoxime groups. As contact time increased, available amidoxime groups were subsided on the absorbent. Exhaustion of available amidoxime groups leaded to the slow kinetic at the later adsorption process. The simulation result was also shown in Fig. 6. The kinetic parameter and correlation coefficient (R 2) were summarized in Table 1. Calculated q e was very close to the experimental qe and the correlation coefficient was found to be 0.97 suggested that pseudo second-order kinetic model represented the adsorption kinetics aptly. Therefore chemisorption was dominated in the uranium sorption by AMCM-0.4 [32].
Effect of uranium concentration and sorption equilibrium
The adsorption isotherm was obtained with different uranium concentrations (50–200 mg L−1) as shown in Fig. 7. Uranium adsorption capacity on AMCM-0.4 increased with increase of initial uranium concentration. The interaction between uranium and AMCM-0.4 was simulated using Non-linear Langmuir and Non-linear Freundlich isotherms described below [33].
Langmuir isotherm:
Freundlich isotherm:
where q m (mg g−1) represents the maximum adsorption capacity, K L is the Langmuir constant, K F is the Freundlich constant and n represents the degree of sorption, respectively.
Non-linear fitting curves were illustrated in Fig. 7. The model parameters were calculated and listed in Table 2. The correlation coefficients (R 2) suggested that experimental data were better simulated by the Langmuir model and the uranium formed a monolayer on the adsorbent surface.
The essential characteristics of the Langmuir isotherm could be expressed in terms of dimensionless separation parameters RL, expressed as Eq. (9):
As shown in Table 3, the R L values for all the tested uranium concentrations were in 0–1 suggesting that AMCM-0.4 was a favorable sorbent for uranium [34].
Desorption studies
Desorption is essential for uranium extracting from aqueous solution. Desorption results were shown in Table 4. Among these tested desorption reagents, HNO3 was the best desorption reagent. Additionally, desorption percentages for 0.1 and 0.5 M were comparable. From the economical point of view 0.1 M HNO3 was the optimum desorption reagent.
Possible sorption mechanism
It is quite difficult to obtain the bonding information only from batch experiments and an assumed model. XPS analyses for AMCM-0.4 before and after uranium sorption (AMCM-0.4-U) were conducted to explore the mechanism of uranium adsorption on AMCM-X. XPS survey spectra were shown in Fig. 8. Two new peaks corresponded to the U 4f7/2(382 eV) and U 4f5/2(392 eV) components in the spectrum of AMCM-0.4-U confirmed that uranium was adsorbed on the surface of AMCM-0.4 [35].
The N 1s and C 1s core-level spectrum of AMCM-0.4 before and after uranium adsorption were shown in Fig. 9. For the uranium loaded adsorbent, the peak of –C=NOH at 399.4 eV shifted to 399.7 eV and the peak of –CH2–NH2 at 400.4 eV shifted to 400.9 eV, indicating that the electron density for the nitrogen atoms decreased. Therefore, it can be deduced that uranium not only interacted with –C=N–OH but also –C–NH2 of the amidoxime group. N atoms were electron donors and uranyl ions were electron acceptors. As shown in C 1s core-level spectrum, the peak of –CH2–CH2– at 284.8 eV unchanged while the peak of 2HN–CH2=NOH at 286.6 shifted to 287.7 eV after sorption. The peak shift of carbon atom in the amidoxime group may be caused by the electron density change of the adjacent nitrogen atoms. The sorption mechanism of uranium on AMCM-X was suggested in Scheme 1.
Uranium adsorption from saline lake brine
The pH of the collected brine samples were 6–7 and the concentration of uranium in the samples were more than 100 times of that in seawater as shown in Table 5. In fact, the concentration of Li+, K+, Na+, Mg2+ were 102–105 times more than the concentration of uranium in the saline lake brine, which was similar to that of seawater [3]. One of the most important aspects in uranium recovery from saline lake brine and seawater was the development of an adsorbent with high uranium sorption rate and selectivity.
Figure 10 shows the uranium adsorption capacity from the saline lake brine by AMCM-0.4. The amount of uranium adsorbed at 1 and 7 days were comparable demonstrated the adsorption reached equilibrium within 1 day. Such fast adsorption kinetics was essential to uranium extraction from saline lake. Great amount of positive ions in saline lake brine compete with uranium for adsorption sites may be the dominant factor for low sorption capacity. The most appropriate adsorbents for uranium extraction from sea water at present were high surface area polymer-based adsorbent, its maximum uranium extraction capacity from seawater was 3.36–3.94 mg g−1. This value was comparable with the uranium sorption capacity on AMCM-0.4 from YT-2 [36].
YT-3, the waste water of potassium extraction from salt lake, was the most economical brine for uranium extraction from salt lake. The selectivity coefficients of amidoximated silica for UO2 2+ in YT-3 were detected. As shown in Table 6, acceptable selectivity coefficients were obtained for uranium with Li, Na, K and Mg even though the initial concentrations of the coexisting ions were extremely high. The results of uranium adsorption from saline lake brine demonstrated that AMCM-0.4 could be a promising adsorbent for uranium extraction from saline lake brine.
Conclusion
Amidoximated silica adsorbents with different amidoxime groups densities were successful prepared and confirmed by FTIR and XPS analysis. Experiments of uranium sorption on AMCM-X were performed. The optimum X value was found to be 0.4. The effect of solution pH on uranium sorption was discussed in detail. Sorption kinetic and isotherms were investigated. The results indicated that chemisorption was dominated in the uranium sorption and the uranium formed a monolayer on the adsorbent surface. XPS spectra of sorbent and uranium loaded sorbent indicated that the sorption was attributed to the interaction between N in amidoxime groups and uranium. Both N atoms of –C=N–OH and –C–NH2 interacted with uranium as electron donors during the sorption. AMCM-X could efficiently absorb the naturally occurring uranium in the saline lake brine. The fast adsorption kinetic and acceptable uranium sorption capacity demonstrated that AMCM-X could be a promising adsorbent for uranium extraction from saline lake brine.
References
Nifenecker H (2011) Future electricity production methods. Part 1: nuclear energy. Rep Prog Phys 74:1–28
Asif M, Muneer T (2007) Energy supply, its demand and security issues for developed and emerging economies. Renew Sust Energ Rev. 11:1388–1413
Kim J, Tsouris C, Mayes RT, Oyola Y, Saito T, Janke CJ, Dai S, Schneider E, Sachde D (2012) Recovery of uranium from seawater: a review of current status and future research needs. Sep Sci Technol 48:367–387
Zhang A, Uchiyama G, Asakura T (2005) PH effect on the uranium adsorption from seawater by a macroporous fibrous polymeric material containing amidoxime chelating functional group. React Funct Polym 63:143–153
Han J, Wang ZM, Hao WL, Lin XB (2011) Preliminary discussions on uranium enrichment in typical saline lakes in Northwestern China. Uranium Geol. 27:160–165
An SW, Wu ZJ (2007) Feasibility of uranium extraction from oilfield waters and salt lake brines in Qaidam basin. J Salt Lake Res. 15:55–62
Liu YL, Yuan LY, Yuan YL, Lan JH, Li ZJ, Feng YX, Zhao YL, Chai ZF, Shi WQ (2012) A high efficient sorption of U(VI) from aqueous solution using amino-functionalized SBA-15. J Radioanal Nucl Chem 292:803–810
Kresge CT, Leonowicz ME, Roth WJ, Vartuli JC, Beck JS (1992) Ordered mesoporous molecular sieves synthesized by a liquidcrystal template mechanism. Nature 359:710–712
Zhao DY, Feng JL, Huo QS, Melosh N, Fredrickson GH, Chmelka BF, Stucky GD (1998) Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 279:548–552
Vidya K, Guptab NM, Selvama P (2004) Influence of pH on the sorption behaviour of uranyl ions in mesoporous MCM-41 and MCM-48 molecular sieves. Mater Res Bull 39:2035–2048
Vidya K, Dapurkar SE, Selvam P, Badamali SK, Gupta NM (2001) The entrapment of UO2 2+ in mesoporous MCM-41 and MCM-48 molecular sieves. Micropor Mesopor Mat. 50:173–179
Chen J, Qu RJ, Zhang Y, Sun CM, Wang CH, Ji CN, Yin P, Chen H, Niu YZ (2012) Preparation of silica gel supported amidoxime adsorbents for selective adsorption of Hg(II) from aqueous solution. Chem Eng J 209:235–244
Tamada M, Seko N, Yoshii F (2004) Application of radiation-graft material for metal adsorbent and crosslinked natural polymer for healthcare product. Radiat Phys Chem 71:223–227
Carboni M, Abney CW, Taylor-Pashow KML, Juan L, Escoto V, Lin WB (2013) Uranium Sorption with Functionalized Mesoporous Carbon Materials. Ind Eng Chem Res 52:15187–15197
Seko N, Katakai A, Tamada M, Sugo T, Yoshii F (2004) Fine fibrous amidoxime adsorbent synthesized by grafting and uranium adsorption–elution cyclic test with seawater. Sep Sci Technol 39:3753–3767
Choi SH, Choi MS, Park YT, Lee KP, Kang HD (2003) Adsorption of uranium ions by resins with amidoxime and amidoxime/carboxyl group prepared by radiation-induced polymerization. Radiat Phys Chem 67:387–390
Liu X, Liu H, Ma H, Cao C, Yu M, Wang Z, Deng B, Wang M, Li J (2012) Adsorption of the uranyl ions on an amidoxime-based polyethylene nonwoven fabric prepared by preirradiation-induced emulsion graft polymerization. Ind Eng Chem Res 51:15089–15095
Gunathilake C, G´orka J J, Dai S, Jaroniec M (2015) Amidoxime-modified mesoporous silica for uranium adsorption under seawater conditions. J Mater Chem A. 21:11650–11659
Zhao YG, Wang XX, Li JX, Wang XK (2015) Amidoxime functionalization of mesoporous silica and its high removal of U(VI). Polym Chem. 30:5376–5384
Bai J, Yin XJ, Zhu YF, Fan FL, Wu XL, Tian W, Tan CM, Zhang X, Wang Y, Cao SW, Fan FY, Qin Z, Guo JS (2016) Selective uranium sorption from salt lake brines by amidoximated Saccharomyces cerevisiae. Chem Eng J 283:889–895
Metilda P, Gladis JM, Rao TP (2004) Influence of binary/ternary complex of imprint ion on the preconcentration of uranium(VI) using ion imprinted polymer materials. Anal Chim Acta 512:63–73
Park E, Condrate RA, Lee D, Kociba K, Gallagher PK (2002) Characterization of hydroxyapatite: before and after plasma spraying. J Mater Sci-Mater M. 13:211–218
Tian YA, Yin P, Qu RJ, Wang CH, Zheng HG, Yu ZX (2010) Removal of transition metal ions from aqueous solutions by adsorption using a novel hybrid material silica gel chemically modified by triethylenetetraminomethylenephosphonic acid. Chem Eng J 162:573–579
Yue DY, Jing Y, Ma J, Xia CL, Yin XJ, Jia YZ (2011) Removal of Neutral Red from aqueous solution by using modified hectorite. Desalination 267:9–15
Beamson G, Briggs D (1992) High Resolution XPS of Organic Polymers: the Scienta ESCA300 Database. Wiley, Chichester
Kallury KMR, Debono RF, Krull UJ, Thompson M (1991) Covalent binding of amino, carboxy, and nitrosubstituted aminopropyltriethoxysilanes to oxidized silicon surfaces and their interaction with octadecanamine and octadecanoic acid studied by X-ray photoelectron spectroscopy and ellipsometry. J Adhesion Sci Technol. 5:801–814
Jin YS, Yan QJ, Yin ZR, Chen Y (1995) Secondary ion mass spectrometry and X-ray photoelectron spectroscopy of Na2MoO4/SiO2catalysts for methane oxidative coupling. J Chem Soc, Faraday Trans 91:381–384
Hong GS, Li X, Shen LD, Wang M, Wang C, Yu XF, Wang XF (2015) High recovery of lead ions from aminated polyacrylonitrile nanofibrous affinity membranes with micro/nano structure. J Hazard Mater 295:161–169
Wang Y, Gu ZX, Yang JJ, Liao JL, Yang YY, Liu N, Tang J (2014) Amidoxime-grafted multiwalled carbon nanotubes by plasma techniques for efficient removal of uranium(VI). Appl Surf Sci 320:10–20
Tian G, Geng JX, Jin YD, Wang CL, Li SQ, Chen Z, Wang H, Zhao YS, Li SJ (2011) Sorption of uranium(VI) using oxime-grafted ordered mesoporous carbon CMK-5. J Hazard Mater 190:442–450
Liu Y, Liu YJ (2008) Biosorption isotherms, kinetics and thermodynamics. Sep Purif Technol 61:229–242
Maziad NA, Abo-Farha SA, Ismail LFM (2009) Radiation induced grafting of glycidylmethacrelate onto polypropylene films for removal of mercury from aqueous solutions. J Macromol Sci A. 46:821–831
Yahaya YA, Mat Don M, Bhatia S (2009) Biosorption of copper(II) onto immobilized cells of Pycnoporus sanguineus from aqueous solution: equilibrium and kinetic studies. J Hazard Mater 161:189–195
Xia CL, Jing Y, Jia YZ, Yue DY, Ma J, Yin XJ (2011) Adsorption properties of congo red from aqueous solution on modified hectorite: kinetic and thermodynamic studies. Desalination 265:81–87
Fan QH, Li P, Chen YF, Wu WS (2011) Preparation and application of attapulgite/iron oxide magnetic composites for the removal of U(VI) from aqueous solution. J Hazard Mater 192:1851–1859
Oyola Y, Janke CJ, Dai S (2016) Synthesis, development and testing of highsurface area polymer-based adsorbents for theselective recovery of uranium from seawater. Ind Eng Chem Res 55:4149–4160
Acknowledgements
This project was financially supported by the National Natural Science Foundation of China (Grant Numbers 11505250, 11575260, 11205216) and the Innovation Key Program of the Chinese Academy of Sciences (Grant Numbers KJCX2-YW-N49-2, KJCX2-YW-N50-3).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yin, X., Bai, J., Tian, W. et al. Uranium sorption from saline lake brine by amidoximated silica. J Radioanal Nucl Chem 313, 113–121 (2017). https://doi.org/10.1007/s10967-017-5283-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-017-5283-1