Abstract
The imidazolium bis(2-ethylhexyl) phosphate moiety was chemically attached on silica gel by chemical modification. The resulting product ([SG-Im]+ [DEHP]−) was characterized by FT-IR spectroscopy, thermogravimetry and elemental analysis. The sorption behavior of Am(III) and Eu(III) on [SG-Im]+ [DEHP]− was studied from dilute nitric acid medium for the separation of Am(III) and Eu(III) from aqueous waste. The effect of time, concentrations of nitric acid and europium in aqueous phase on the distribution coefficient (K d) was studied. The study indicated the possibility of using modified silica for the separation of Eu(III) from Am(III) with high separation factors (>50 at 0.1 M HNO3).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Room temperature ionic liquids (RTILs) are organic salts melt at temperatures lower than 100 °C and they are finding several applications in the separation of toxic and radiotoxic metal ions from wide variety of aqueous feeds [1,2,3,4,5]. In liquid–liquid extraction, RTILs are being employed as an alternative to molecular volatile organic compounds and n-paraffins. The RTIL diluents in conjunction with the conventional ligands were shown to exhibit extraordinary extraction of toxic metal ions from aqueous phase. Several authors have exploited this feature and explored the possibility of using ionic liquids for solvent extraction applications [6,7,8,9,10,11].
The employment of ionic liquids in solvent extraction has some drawbacks. Since RTILs are comprised entirely of ions they exhibit significant solubility in aqueous phase. The traditional imidazolium bis (trifluoromethane sulphonyl)imide ionic liquids are not suitable for solvent extraction applications, as the main pathway for metal ions extraction is by cation exchange [12]. In addition, the bis(triflouromethanesulphonyl) imide anion also undergo anion exchange [13] with various anions present in aqueous phase. This exchange reaction often results in the pollution of aqueous phase and issues also develop with respect to the loss of room temperature ionic liquid molecules from organic phase during recycling. Therefore, there is a need to develop advanced ionic liquid based systems for the extraction of metal ions from aqueous medium.
The problem of ionic liquid loss [14] to aqueous phase can be minimized by anchoring the ionic liquid moiety on solid supports [15,16,17,18,19,20] such as silica gel, and organic polymers. Among the cation and anionic moiety of the ionic liquid, anchoring of cationic moiety on inert solid supports is relatively easy. The anion can be chosen in such a way that it is strongly hydrophobic and insoluble in aqueous phase. In this connection, Xin et al. reviewed [21] the use of ionic liquid anchored on solid supports for various applications. Yang et al. [22] developed a magnetic microsphere confined ionic liquid as a novel sorbent for the determination of chlorophenols in environmental water samples. Galán-Cano et al. [23] studied the use of ionic liquid coated magnetic nanoparticles for the gas chromatography/mass spectrometric determination of polycyclic aromatic hydrocarbons in waters. Similarly, Fontanals et al. [24] studied the extraction of a group of acidic compounds from complex aqueous samples by polymer-supported imidazolium trifluoroacetate salt. In this context, we also developed ionic liquid anchored on magnetic adsorbents [25] for the separation of Am(III) and Eu(III) from dilute nitric acid medium. In this paper, we report anchoring of imidazolium bis(2-ethylhexyl)phosphate on the inert matrix, silica gel, and studies on the extraction of Am(III) and Eu(III) from dilute nitric acid medium. The imidazolium moiety was covalently linked to silica gel by organo modification reaction on silica gel surface. Since the anionic moiety bis(2-ethylhexyl)phosphate was insoluble in aqueous phase, it does not undergo anion exchange with anions present in aqueous phase. In the present study, Am(III) was used as trivalent actinide representative and Eu(III) was used as lanthanide representative for extraction studies.
Experimental
Instrumentation
The Fourier transformed infrared (FTIR) spectrum of the sample was recorded using BRUKER TENSOR II FT-IR spectrometer equipped with an ATR (attenuated total reflectance) diamond crystal and Zn–Se window. Proton NMR analysis was done using Bruker AVANCE III 500 MHz (AV 500) multi nuclei solution NMR. TGA pattern was recorded by subjecting the sample at a heating rate of 10 K min−1 for the temperature range of 298–873 K by using thermogravimetric analyzer model number TGA/SDTA 851e of M s−1. Mettler Toledo GmbH, Switzerland. Microelemental CHNS analysis of the adsorbent was carried out by using Elementer Vario-EL.
Materials
N-Methylimidazole and 3-chloropropyltriethoxysilane were purchased from Alfa Aesar. Bis(2-ethylhexyl)phosphoric acid (HDEHP) and chloroform were obtained from Merck. The chemicals like isopropanol, acetone were analytical grade and they were used without any further purification. Chromatographic silica gel of particle size 60–120 mesh was purchased from E-Merck. The silica gel (20 g) was stirred with nitric acid (8 M, 100 mL) at room temperature for 2 h. The mixture was then refluxed for 8 h at 100 °C. The silica gel was filtered and washed with distilled water, until the pH of the filtrate was ~7. The activated silica gel was washed with surplus water and air dried over night. The silica gel obtained after air drying was used for anchoring.
Preparation of [SG-Im]+ [DEHP]−
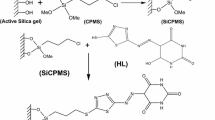
The imidazolium bis(2-ethylhexyl) phosphate was chemically attached on the surface of the silica gel by the reaction scheme shown in Fig. 1. The systematic procedure is described below.
Preparation of sodium bis(2-ethylhexyl)phosphate (Na-DEHP)
Bis(2-ethylhexyl)phosphate (15 mmol) in chloroform was refuxed with sodium hydroxide (18 mmol) for about 6 h at 60 °C. The product obtained was separated from aqueous phase (sodium hydroxide) and chloroform was removed by rotary evaporation. The final product was dried under high vacuum. A pale yellow viscous liquid (Na-DEHP) was obtained with 98% yield.
Preparation of 1-methyl-3 (3-ethoxysilylpropyl) imidazolium chloride (ImSi-Cl)
Equimolar quantity of N-methylimidazole (120 mmol) was refluxed with 3-chloropropyltriethoxysilane (120 mmol) for 48 h at 80 °C. The product obtained was washed with diethyl ether thoroughly and dried under vacuum. A pale yellow viscous liquid (ImSi-Cl) was obtained. The product obtained after drying was characterized by 1H NMR (Bruker Avance III 500 MHz) and FT-IR. 1H NMR (CDCl3, δ/ppm relative to TMS): 0.628–0.589 (2H, t, –CH2–Si), 1.228–1.193 (9H, t), 2.024–2.006 (2H, m), 3.837–3.788 (6H, q), 4.111 (3H, s, –N–CH3), 4.346–4.311 (2H, m), 7.295 (1H, s), 7.515 (1H, s, –N–CH–), 10.644 (1H, s, –N–CH–N–). FT-IR shows following the characteristic bands: 2962 cm−1 (C–H str), 1456 cm−1 (C–N str), 1568 cm−1 (C–C str), 1300 cm−1 (Si–C str), 1098 cm−1 (Si–O str), 1136 (C–O str). The yield of 1-methyl-3 (3-ethoxysilylpropyl) imidazolium chloride (ImSi-Cl) was 82.05%.
Preparation of 1-methyl-3(3-ethoxysilylpropyl)imidazolium bis(2- ethylhexyl)phosphate (ImSi-DEHP)
ImSi-Cl (20 mmol) in chloroform (50 mL) was stirred with NaDEHP (20 mmol) for 24 h at room temperature. The precipitated NaCl was separated by filteration. The product obtained (ImSi-DEHP) was washed with small amount of water to remove excess NaCl, then chloroform was removed under reduced pressure and dried under vacuum to obtain a highly viscous yellow liquid. The product obtained after drying was characterized by 1H NMR (Bruker Avance III 500 MHz) and FT-IR. 1H NMR (CDCl3, δ/ppm relative to TMS): 0.572 (2H, t, –CH2–Si), 0.868–0.819 (12H, m), 1.245–1.217 (23H, m), 1.485–1.360 (6H, m), 3.985 (3H, s, –N–CH3), 3.731–3.677 (10H, m, –O–CH2), 4.264–4.250 (2H, t, –N–CH2), 7.617 (1H, s), 9.628 (1H, s, –N–CH–), 9.794 (1H, s, –N–CH–N–). FT-IR showed following the characteristic bands: 2965 cm−1 (C–H str), 1454 cm−1 (C–N str), 1569 cm-1 (C–C str), 1308 cm−1 (Si–C str), 1102 cm−1 (Si–O str), 1131 cm−1 (C–O str), 1040 cm−1 (P=O str and P–O–C out of phase str). The yield of the product obtained in this step was 87.8%.
Immobilization of ionic liquid on silica
ImSi-DEHP (5 mmol) was dissolved in chloroform (30 mL). Activated silica (3 g) was added to ImSi-DEHP in chloroform and refluxed for 24 h at 65 °C. The newly modified [SG-Im]+ [DEHP]− was filtered and washed with chloroform, isopropanol and acetone. The [SG-Im]+ [DEHP]− adsorbent was dried under ambient condition overnight. This was used for all extraction studies.
Extraction of Am(III) and Eu(III)
All equilibration experiments were performed at 298 K. Extraction efficiency of Am(III) and Eu(III) in [SG-Im]+ [DEHP]− was determined by measuring the distribution coefficients of Am(III) and Eu(III). The experiment involved equilibration of 0.05 g of the sorbent with 10 mL of nitric acid (concentration varied from 0.001 to 0.1 M) spiked with the corresponding radiotracers (~10−4 M, 241Am tracer was used for Am(III) and (152+154)Eu tracer was used for Eu(III)) in 20 mL stoppered glass test-tubes. The test-tubes were rotated by using a test-tube rotator, which rotates in up-down manner with a constant speed of 50 rpm for a specified period of time. The radioactivites of 241Am(III) and 152+154Eu(III) before and after equilibration were determined by radiation counting technique using NaI(Tl) single channel analyser. The distribution coefficient (K d) of Am(III) and Eu(III) was determined from the radioactivity measurement of aqueous phase using Eq. 1. The equilibration experiment was performed in triplicate and the radioactivity of the sample in each solution was counted five times for a specific period of time to minimize the experimental and statistical error. The error in the distribution coefficient was found to be ±5%. The separation factor (SF) was determined using Eq. 2.
where A 0 and A f are the initial and final radioactivity (counts per minute per mL of the sample) of aqueous phase. V is the volume of aqueous phase, and m is the mass of the sorbent taken for equilibration.
Rate of uptake of Am(III) (or Eu(III)) was studied by equilibrating 0.05 g of sorbent with 10 mL of nitric acid (0.001 M), spiked with 241Am(III) tracer (or (152+154)Eu(III) in the case of Eu(III) study) at 298 K. Separate experiments were performed for 2–360 min. Aliquots were drawn from the aqueous phase before and after equilibration. From the initial radioactivity and the radioactivity measured at various intervals of time, the percentage extraction of Am(III) (or Eu(III) extracted was calculated by using Eq. 3.
Adsorption isotherm
Adsorption isotherm of Eu(III) on [SG-Im]+ [DEHP]− was studied by equilibrating 0.05 g of the sorbent with 10 mL of nitric acid solution containing various quantities of europium nitrate spiked with (152+154)Eu(III) tracer. Equilibration experiments were carried out for 2 h at 298 K. The amount of Eu(III) extracted was measured from radioactivity values of the aqueous solution before and after equilibration as discussed above.
Results and discussions
Characterization of the sorbent
The elemental analysis of the sorbent, [SG-Im]+ [DEHP]−, indicated the presence of 7.1% C and 1.12% nitrogen. Based on the elemental analysis of nitrogen, the amount of imidazolium bis(2-ethylhexyl) phosphate on silica gel was determined to be 0.4 mmol g−1. Thermogravimetry pattern of silica gel and [SG-Im]+ [DEHP]− are shown in Fig. 2. It can be seen that the weight loss occurring below 100 °C in both cases is due to the loss of loosely bound water molecules especially adsorbed on the surface of silica gel as well as [SG-Im]+ [DEHP]−. However, in case of [SG-Im]+ [DEHP]− the loss of water molecules is gradual and require more temperature (~150 °C) for completion, as shown in Fig. 2. This could be due to the ionic nature of surface which holds the water molecules strongly and require higher temperature for vaporisation. The weight loss occurring in the temperature range 200–800 °C in case of silica gel is very gradual and this could be attributed to the cross condensation of silanol group present on the surface of silica gel leading to the continuous loss of water molecules. Since the energy of silanol groups present on the surface of silica gel is not uniform, the condensation occurs at different temperatures, resulting in a gradual but continuous decrease in the weight of the sample. In contrast to this, the weight loss occurring in the temperature range 200–600 °C is more and this can be attributed to the loss of organics present on the surface of [SG-Im]+ [DEHP]−. This observation confirms the presence of organic moiety on the surface of silica gel. Moreover the elemental analysis and IR pattern confirmed the presence of imidazolium phosphate moiety on the surface of the silica gel.
Rate of sorption
The percentage of sorption of Am(III) and Eu(III) by [SG-Im]+ [DEHP]− as a function of time is shown in Fig. 3. It is observed from the figure that the extraction of Am(III) and Eu(III) on [SG-Im]+ [DEHP]− increases with increase in time, the percentage of sorption reaches a saturation after 100 min of equilibration. The variation in the rate of sorption of Am(III) and Eu(III) after 4 h of equilibration is insignificant. Therefore, all the sorption experiments were performed for a duration of 4 h to ensure the establishment of equilibrium. Figure 3 also shows the fitted curves for the percentage extraction of Am(III) and Eu(III) by pseudo-first order (dotted line) and pseudo-second order (solid line) kinetic models. The derived equations for first order and second order kinetic models are shown in Eqs. 4 and 5. The detailed derivations of these equations are discussed elsewhere [26]. It should be noted that these equations were derived for homogeneous systems. However, several researchers utilized these equations for describing the heterogeneous (solid–liquid phase) extraction systems and for calculating the rate constant and other parameters [27,28,29,30,31]
where q t is the concentration of metal ions adsorbed at a time t (in terms of % sorption) and q eq is the concentration of metal ions adsorbed at equilibrium and k 1 and k 2 are the rate constants of pseudo first order and pseudo second order rate constants respectively. The fitting parameters and rate constants of both the first order and second order kinetic models are also provided in Fig. 3. It can be seen from fitting parameters, χ 2 and R 2 values, that the rate of sorption of Am(III) and Eu(III) is well described by the pseudo-second order kinetic model. The second order rate constant for Am(III) and Eu(III) obtained from the fitting are 0.03 and 0.01 L min−1 mol−1 respectively.
Distribution coefficients of Am(III) and Eu(III)
Figure 4 shows the sorption behavior of Am(III) and Eu(III) as a function of nitric acid concentration at equilibrium in aqueous phase. It can be seen that the distribution coefficient of Am(III) and Eu(III) decreases with increase in the equilibrium concentration of nitric acid, indicating the involvement of cation exchange mechanism. The K d value decreases from ~60,000 to 5 mL g−1 in case of Eu(III) and from ~40,000 to 0.1 mL g−1 in case of Am(III) with increase of nitric acid concentration from 0.001 M to 0.1 M. The separation factor (=K d of Eu(III) over Am(III)) determined from the distribution ratios and they are tabulated in Table 1. It can be seen that the separation factor of Eu(III) over Am(III) decreases with decrease in the concentration of nitric acid. At 0.1 M nitric acid a SF of about 50 was achieved. It should be noted that the separation factors were achieved without the addition of any complexing reagents to aqueous phase. The complexing agents are generally necessary to achieve a reasonable Am/Eu separations. However, the present study shows that the separation factor of ~50 at 0.1 M nitric acid can be achieved without any complexing agent using [SG-Im]+ [DEHP]−.
Based on this observation the following mechanism can be proposed for the extraction of Am(III) and Eu(III) in [SG-Im]+ [DEHP]−. The sorbent [SG-Im]+ [DEHP]− reacts with nitric acid to yield [SG-Im]+ [NO3 −]ad and HDEHPad by the reaction shown in Eq. 6. The subscript “ad” and “aq” indicates the sorbent and aqueous phase respectively. It should be noted that the products [SG-Im][NO3]ad and [HDEHP]ad are present the sorbent phase. The acid HDEHP formed is a weak phosphoric acid and insoluble in aqueous phase. Therefore HDEHP is held in the adsorbed phase. HDEHP is a well-known reagent for the extraction of Am(III) and Eu(III) by cation exchange mechanism. Linear regression analysis of the plot of Kd versus [HNO3] resulted a slope of approx. −3 (see Fig. 4) for both Eu(III) and Am(III) extraction in \(\left[ {\text{SG - Im}} \right]_{\text{ad}}^{ + }\) \(\left[ {\text{DEHP}} \right]_{\text{ad}}^{ - }\). This shows that the sorbent releases three moles of H+ ions for the extraction of a mole of Eu(III) or Am(III). Based on this observation, the following mechanism (Eqs. 6 and 7) can be proposed for the extraction of trivalent metal ion.
The equilibrium constant for the above reaction can be represented by Eq. 8.
But the distribution coefficient of metal in sorbent phase is defined by Eq. 1 and the same is shown in Eq. 9.
Upon combining Eqs. 8 and 9 and converting to logarithmic form,
where K′ is a constant at a constant sorbent concentration. At low nitric acid concentrations (lower than 0.003 M), there is a deviation from the rest of the distribution coefficients as shown in Fig. 4. This could be due to the involvement of some other mechanism operating for the extraction of Am(III) and Eu(III), which requires more detailed investigations.
Adorption isotherm
The Langmuir adsorption isotherm for the sorption of Eu(III) on [SG-Im]+ [DEHP]− at 0.001 M nitric acid is shown in Fig. 5. It can be seen that the amount of Eu(III) loaded in the sorbent phase increases with increase in the amount of metal ion present in aqueous phase. The linear Langmuir adsorption model is described by Eq. 13.
where C f is the concentration of the metal ion in the aqueous phase and Cs is the concentration of the metal ion in the sorbent phase. K L is Langmuir constant (in L mg−1) and b is the apparent sorption capacity (in mg g−1). Linear regression analysis of the sorption data results in the determination of apparent sorption capacity and Langmuir constant. The value of apparent sorption capacity of Eu(III) sorption on [SG-Im]+ [DEHP]− was determined to be 7 mg g−1, which is lower than the sorption capacity determined (20 mg g−1) by elemental analysis of nitrogen on [SG-Im]+ [DEHP]−. The value of K L was determined to be 0.13 L mg−1. Lower apparent sorption capacity observed in [SG-Im]+ [DEHP]− could be due to the presence of nitric acid in aqueous phase that seems to shift the equilibrium reaction shown in Eqs. 6 and 7 in backward direction.
Conclusion
A new imidazolium bis(2-ethylhexyl)phosphate modified slica gel, [SG-Im]+ [DEHP]− was prepared and studied for the sorption of americium(III) and europium(III) from dilute nitric acid medium. Pseudo-second order rate of extraction for Am(III) and Eu(III) was observed. The extraction equilibrium was established in 2 h. The distribution coefficient of Am(III) and Eu(III) decreased with increase in the concentration of nitric acid. The slope analysis of extraction data, indicated the release of three H+ ions for the extraction of a trivalent metal ion in the acid range 0.001–0.1 M.
The separation factor of Eu(III) over Am(III) decreased with decrease in the concentration of nitric acid. At 0.1 M nitric acid a SF of about 50 was achieved. It was noted that the separation factor was achieved without the addition of any complexing reagents to aqueous phase, which was usually required for lanthanide-actinide separations. Therefore, the study confirmed the feasibility of separating Am(III) from Eu(III) from dilute nitric acid by using [SG-Im]+ [DEHP]− without any complexing agents present in aqueous phase.
References
Raut DR, Mohapatra PK (2015) Extraction of uranyl ion using 2-thenoyltrifluoro acetone (HTTA) in room temperature ionic liquids. Sep Sci Technol 50:380–386
Vasudeva Rao PR, Venkatesan KA, Rout A, Srinivasan TG, Nagarajan K (2012) Potential applications of room temperature ionic liquids for fission products and actinide separation. Sep Sci Technol 47:204–222
Mohapatra PK, Sengupta A, Iqbal M, Huskens J, Verboom W (2013) Diglycolamide-functionalized calix [4] arenes showing unusual complexation of actinide ions in room temperature ionic liquids: role of ligand structure, radiolytic stability, emission spectroscopy, and thermodynamic studies. Inorg Chem 52:2533–2541
Priya S, Sengupta A, Jayabun S, Adya VC (2016) Piperidinium based ionic liquid in combination with sulphoxides: highly efficient solvent systems for the extraction of thorium. Hydrometallurgy 164:111–117
Li C, Wu L, Chen L, Yuan X, Cai Y, Feng W, Liu N, Ren Y, Sengupta A, Murali MS, Mohapatra PK (2016) Highly efficient extraction of actinides with pillar [5] arene-derived diglycolamides in ionic liquids via a unique mechanism involving competitive host–guest interactions. Dalt Trans 45:19299–19310
Wei GT, Yang Z, Chen CJ (2003) Room temperature ionic liquid as a novel medium for liquid/liquid extraction of metal ions. Anal Chim Acta 488(2):183–192
Marsh KN, Deev A, Wu AC, Tran E, Klamt A (2002) Room temperature ionic liquids as replacements for conventional solvents: a review. Korean J Chem Eng 19(3):357–362
Nakashima K, Kubota F, Maruyama T, Goto M (2005) Feasibility of ionic liquids as alternative separation media for industrial solvent extraction processes. Ind Eng Chem Res 44(12):4368–4372
Visser AE, Swatloski RP, Reichert WM, Griffin ST, Rogers RD (2000) Traditional extractants in nontraditional solvents: groups 1 and 2 extraction by crown ethers in room-temperature ionic liquids. Ind Eng Chem Res 39(10):3596–3604
Huddleston JG, Willauer HD, Swatloski RP, Visser AE, Rogers RD (1998) Room temperature ionic liquids as novel media for ‘clean’liquid–liquid extraction. Chem Commun 16:1765–1766
Marsh KN, Deev A, Wu AC, Tran E, Klamt A (2002) Room temperature ionic liquids as replacements for conventional solvents: a review. Korean J Chem Eng 19(3):357–362
Visser AE, Swatloski RP, Reichert WM, Mayton R, Sheff S, Wierzbicki A et al (2002) Task-specific ionic liquids incorporating novel cations for the coordination and extraction of Hg2+ and Cd2+: synthesis, characterization, and extraction studies. Environ Sci Technol 36(11):2523–2529
Jensen MP, Neuefeind J, Beitz JV, Skanthakumar S, Soderholm L (2003) Mechanisms of metal ion transfer into room-temperature ionic liquids: the role of anion exchange. J Am Chem Soc 125(50):15466–15473
Wellens S, Thijs B, Binnemans K (2012) An environmentally friendlier approach to hydrometallurgy: highly selective separation of cobalt from nickel by solvent extraction with undiluted phosphonium ionic liquids. Green Chem 14(6):1657–1665
Li DY, Wang YZ, Zhao XL, He XW, Li WY, Zhang YK (2014) Facile synthesis of ionic liquid functionalized silica-capped CdTe quantum dots for selective recognition and detection of hemoproteins. J. Mater. Chem. B 2(34):5659–5665
Mehnert CP, Cook RA, Dispenziere NC, Afeworki M (2002) Supported ionic liquid catalysis: a new concept for homogeneous hydroformylation catalysis. J Am Chem Soc 124(44):12932–12933
Luo S, Mi X, Zhang L, Liu S, Xu H, Cheng JP (2006) Functionalized chiral ionic liquids as highly efficient asymmetric organocatalysts for Michael addition to nitroolefins. Angew Chem Int Ed 45(19):3093–3097
Kim DW, Roshan R, Tharun J, Cherian A, Park DW (2013) Catalytic applications of immobilized ionic liquids for synthesis of cyclic carbonates from carbon dioxide and epoxides. Korean J Chem Eng 30(11):1973–1984
Li H, Bhadury PS, Song B, Yang S (2012) Immobilized functional ionic liquids: efficient, green, and reusable catalysts. RSC Adv 2(33):12525–12551
Xin B, Hao J (2014) Imidazolium-based ionic liquids grafted on solid surfaces. Chem Soc Rev 43(20):7171–7187
Vidal L, Riekkola ML, Canals A (2012) Ionic liquid-modified materials for solid-phase extraction and separation: a review. Anal Chim Acta 715:19–41
Fei Y, Rui S, Yiming L, Xiangyu S, Fei T, Qingyun C, Shouzhuo Y (2011) Magnetic microsphere confined ionic liquid as a novel sorbent for the determination of chlorophenols in environmental water samples by liquid chromatography. J Environ Monit 13:440–445
Galán-Cano F, del Carmen AM, Lucena R, Cárdenas S, Valcárcel M (2013) Ionic liquid coated magnetic nanoparticles for the gas chromatography/mass spectrometric determination of polycyclic aromatic hydrocarbons in waters. J Chromatogr A. 1300:134–140
Fontanals N, Ronka S, Borrull F, Trochimczuk AW, Marcé RM (2009) Supported imidazolium ionic liquid phases: a new material for solid-phase extraction. Talanta 80(1):250–256
Suneesh AS, Kumaresan R, Jain R, Venkatesan KA, Antony MP, Bhanage BM (2016) A magnetic adsorbent for the mutual separation of Am(III) and Eu(III) from dilute nitric acid medium. Colloids Interface Sci Commun 12:13–16
Reddad Z, Gerente C, Andres Y, Le Cloirec P (2002) Adsorption of several metal ions onto a low-cost biosorbent: kinetic and equilibrium studies. Environ Sci Technol 36(9):2067–2073
Tan IAW, Hameed BH, Ahmad AL (2007) Equilibrium and kinetic studies on basic dye adsorption by oil palm fibre activated carbon. Chem Eng J 127(1):111–119
Ho YS, McKay G (1998) The kinetics of sorption of basic dyes from aqueous solution by sphagnum moss peat. Can J Chem Eng 76(4):822–827
Tan IAW, Ahmad AL, Hameed BH (2009) Adsorption isotherms, kinetics, thermodynamics and desorption studies of 2, 4, 6-trichlorophenol on oil palm empty fruit bunch-based activated carbon. J Hazard Mater 164(2):473–482
Yousef RI, El-Eswed B, Ala’a H (2011) Adsorption characteristics of natural zeolites as solid adsorbents for phenol removal from aqueous solutions: kinetics, mechanism, and thermodynamics studies. Chem Eng J 171(3):1143–1149
Ngah WW, Hanafiah MAKM (2008) Adsorption of copper on rubber (Hevea brasiliensis) leaf powder: kinetic, equilibrium and thermodynamic studies. Biochem Eng J 39(3):521–530
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dasthaiah, K., Robert Selvan, B., Suneesh, A.S. et al. Ionic liquid modified silica gel for the sorption of americium(III) and europium(III) from dilute nitric acid medium. J Radioanal Nucl Chem 313, 515–521 (2017). https://doi.org/10.1007/s10967-017-5314-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-017-5314-y