Abstract
The gamma degradation of toxic non-oxidizing biocide dodecyl dimethyl benzyl ammonium chloride (DDBAC) was investigated. The degradation of DDBAC achieved 70–100% depending on the initial concentration and the absorbed dose, but only 10–33% dissolved organic carbon was removed. The presence of NO3 −, HCO3 −, 2-propanol and tert-butanol inhibited the degradation of DDBAC. The DDBAC degradation rate constant ratios of ·OH, ·H and e −aq was calculated as 7.4:1.4:1. The acute toxicity of 10 mg L−1 DDBAC was removed by 60% at absorbed doses of 0.5–3.0 kGy. The results showed that gamma irradiation was effective to remove DDBAC and its toxicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As an advanced membrane technology, reverse osmosis (RO) is now widely used in water desalination, potable water production and tertiary treatment of wastewater. With the recovery ratios ranging from 35 to 85%, a large volume of brines are generated during RO processes [1]. The concentrate always contains high concentration of salts, organic pollutants and other chemical agents used to prevent membrane fouling, such as antiscalants and antibiofouling agents, which can cause negative environmental impacts from uncontrolled brine discharges [2]. Especial attention should be paid to the additive chemical agents during the RO processes. For example, the non-oxidizing biocides (i.e., Dodecyl dimethyl benzyl ammonium chloride, DDBAC) are effective for biofouling control without deteriorating RO membranes [3]. However, such chemicals are always non-biodegradable and toxic to organisms and humans [4, 5]. Effective removal of such chemicals from the RO brine are necessary before the discharge.
Advanced oxidation processes (AOPs) are effective to reduce the organic concentration in RO brines and breakdown toxic and refractory pollutants, such as pharmaceuticals and pesticides, which improves the biodegradability of RO brines simultaneously [1, 6]. The commonly used AOPs include ozonation, fenton process and photooxidation [6, 7]. However, these technologies are not so effective for certain pollutants, and with disadvantages of high energy and chemical consumption. Furthermore, the chemicals added in AOPs may cause new problems during the following wastewater treatment. The irradiation by 60Co source and electron beam accelerator has been recognized as a promising way to destroy the refractory pollutants. On one hand, gamma rays impose direct action to pollutants [8]. On the other hand, gamma irradiation can generate reactive species (e.g., hydroxyl radicals, hydrated electron and hydrogen atoms) through ionizing water molecules to degrade the organic pollutants [9]. Previous studies have shown that gamma irradiation can degrade many refractory pollutants effectively, such as pharmaceutical and personal care products, phenolic and chlorophenols and herbicides [10,11,12]. However, there is little knowledge on the radiolytic degradation of refractory substances in the RO brines.
In this study, the degradation of a non-oxidizing biocide DDBAC in aqueous solutions was investigated with gamma irradiation using a 60Co source. The effects of initial DDBAC concentration, pH value, irradiation dose, and some additives on the degradation of DDBAC were systematically studied. The degradation kinetics were calculated and compared for different conditions. Finally, the changes of acute toxicity of DDBAC solution during gamma irradiation process were determined using a photo-bacterium bioassay method.
Experimental
Chemicals
Chemicals and materials
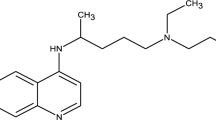
DDBAC (purity >99%) was bought from J&K Scientific, Ltd. (Beijing, China), and the structure was shown in Fig. 1. All other chemicals were of analytical grade. Sodium dihydrogen phosphate dihydrate (NaH2PO4), sodium carbonate anhydrous (NaHCO3) and phosphoric acid (H3PO4) were bought from Peking Reagent (Beijing, China). Disodium hydrogen phosphate dodecahydrate (Na2HPO4) and sodium nitrate (NaNO3) were bought from Xilong Scientific (Shantou, China). Tert-butanol and 2-propanol were bought from Tianjin Guangfu Fine Chemical Research Institute (Tianjin, China). All the solutions were prepared by ultrapure water (resistivity >18 MΩ cm) using a Mili-Q device (Integral 5, Milipore, U.S.).
Experimental process
A 60Co source at the Institute of Nuclear and New Energy Technology (INET), Tsinghua University, Beijing China was applied to perform the gamma irradiation experiments at 25 °C. The samples of 25 mL were preserved in 25 mL colorimetric quartz tubes and placed at the marginal channel of the cobalt source. The irradiation dose rate was 0.5 Gy s−1 measured using “Standard method for using the ferrous sulfate (Fricke) dosimeter to measure absorbed dose in water (GB/T 139-2008)” with uncertainty of below 5%. The cumulated energy absorbed in the sample was proportional to the exposure time. Based on preliminary experiments, seven absorbed doses including 0, 0.2, 0.5, 1.0, 1.5, 2.5 and 3.0 kGy were tested. All the samples were prepared at least in duplicate.
The DDBAC solutions were prepared by dissolving certain amounts of DDBAC in 10 mmoL phosphate buffer (Na2HPO4 and NaH2PO4) for pH 7 solutions. The buffer was used to keep the pH value steady during the degradation experiments. Four sets of experiments were carried out as follows.
-
1.
To test the effects of initial DDBAC concentrations, three concentrations of DDBAC solutions including 10, 50 and 100 mg L−1 were prepared, which were all below the critical micelle concentration of DDBAC (ca. 340 mg L−1). The initial pH values were all 7.0.
-
2.
To test the effects of initial pH values, three solutions of 50 mg L−1 DDBAC were prepared with pH values of 3, 7 and 11, respectively. The solutions of pH 3 were adjusted using H3PO4 and NaH2PO3. The solutions of pH 11 were adjusted using NaOH and Na2HPO3.
-
3.
To test the effects of inorganic salts, 0.01 mol L−1 NaNO3 or 0.01 mol L−1 NaHCO3 was added to the solution of 50 mg L−1 DDBAC.
-
4.
To test the effects of organic matters, 0.01 mol L−1 2-propanol or 0.01 mol L−1 tert-butanol was added to the solution of 50 mg L−1 DDBAC.
After irradiation, the concentrations of DDBAC, dissolved organic carbon (DOC) and pH values in the solutions were determined.
Analytical methods
All water samples were first filtered by 0.45 μm filters before analysis. DDBAC concentration was measured by a high performance liquid chromatography (HPLC, LC-20 AT, Shimadzu) at 254 nm with an ODS-C8 column (250 × 4.6 mm, 5 μm particle size, JK Chemical Co., China). Two mobile phases were used, (A) acetonitrile and (B) 10 mmol L−1 phosphate solution with 0.1% formic acid. The volume ratio of mobile phase A to B was 55:45, and the flow rate was 0.8 mL min−1. The column temperature was 40 °C. The injection volume of each sample was 20 μL and the detection limit was 1.0 mg L−1. The concentration of DOC in each sample was measured by a total organic carbon analyzer (TOC-VCPH, Shimadzu, Japan). The pH values of solutions before and after irradiation were measured using a pH meter (PHS-3G, Leici Corp., China).
A photo-bacterium bioassay method was used to determine the acute biotoxicity of the DDBAC solutions. The light emission of the bioluminescent bacteria (Photobacterium phosphoreum) will decrease when expose to toxic matters [13]. Bioluminescence after a 15-min exposure to different solutions were determined. The bioluminescence inhibition ratio was calculated as L/L 0, where L 0 was the bioluminescence of bacterial suspension, and L was the bioluminescence of bacterial suspension exposed to DDBAC solution.
Data analysis
The relative concentration was calculated as C/C 0, where C 0 (mg L−1) was the initial concentration of DDBAC, and C (mg L−1) was the concentration of DDBAC in the irradiated solution. The relative DOC concentration was calculated as DOC/DOC0, where DOC0 (mg L−1) was the initial DOC concentration of the DDBAC solution, and DOC (mg L−1) was the DOC concentration in the irradiated solution.
The radiation chemical yield (G-value) is defined as the number of species formed or decomposed in solution when one Joule energy is absorbed [14]. G-value (μmol J−1) was calculated as G = (C 0−C)/D × 106, where C 0 (mol L−1) and C (mol L−1) were the DDBAC concentrations at the initial moment and at an absorbed dose of D (Gy), respectively.
Results and discussion
Effect of initial concentration on DDBAC radiolysis
The effect of initial concentration on the radioactive degradation of DDBAC was shown in Fig. 2. DDBAC at a lower initial concentration was more easily degraded at a given absorbed dose. For DDBAC solution of 10 mg L−1, the concentration of DDBAC was reduced to lower than the detected limit at an absorbed dose of 0.2 kGy, which showed that gamma irradiation can degrade DDBAC efficiently. The removal ratios of DDBAC at an absorbed dose of 0.5 kGy were 73 and 46% for the initial concentrations of 50 and 100 mg L−1, respectively. The data (−ln (C/C0)) was linearly correlated with the absorbed dose (inset of Fig. 2), indicating that the degradation kinetics of DDBAC fitted well with the pseudo first-order reaction kinetics. The apparent reaction rate constants (k) and the correlation coefficients (R 2) under different conditions were calculated (Table 1). The k values were 3.03 and 1.36 kGy−1 for the initial DDBAC concentrations of 50 and 100 mg L−1, respectively, which decreased significantly with the increase of initial DDBAC concentration. These results were consistent with previous reports using other pollutants [8, 14, 15]. It is possible that the reactive species were generated at a constant rate due to the water radiolysis, which became limited with the increase of the initial DDBAC concentration and led to the decrease of removal ratio.
The mineralization of DDBAC during the radiolysis process was measured using the variation of DOC (Fig. 3). The DOC values gradually decreased with the increasing absorbed dose. The DOC reduction ratios ranged from 10 to 33% for 10–100 mg L−1 DDBAC solutions, which were much smaller than the removal ratios of DDBAC. The results indicated that DDBAC was more easily degraded to intermediates than total mineralization under the experimental conditions. However, DDBAC can be further mineralized when increasing the absorbed dose. For 50 mg L−1 DDBAC solution, the DOC reduction ratio reached 80% at an absorbed dose of 10 kGy, and the ratio kept increased to 88% as the absorbed dose increased to 30 kGy. But the energy cost will increase by a large margin simultaneously. Similar to the results in Fig. 2, the DOC had a more significant reduction for the lower initial concentration of DDBAC.
Effect of initial pH value on DDBAC radiolysis
The effect of initial pH values on DDBAC radiolysis was investigated (Fig. 4). The three curves of DDBAC were basically coincident. The kinetics constants (k) were 3.13, 3.03 and 2.81 kGy−1 for initial pH values of 3, 7, and 11, respectively. Only a slightly lower k value was observed under the alkaline condition. The irradiation chemical yield G-values at an absorbed dose of 1 kGy were all 0.14 μmol J−1 for the three pH conditions (Table 1). These data all showed that the initial pH values (3–11) of the solution had little effect on the degradation of DDBAC. The pH values kept steady during irradiation. These results were different from the previous reports, where lower efficacy of irradiation was observed under alkaline conditions (pH ≤11) due to the transformation of hydroxyl radicals into the less reactive ·O− [11, 14, 16]. It is possible that DDBAC is easily degraded by various active groups, including hydroxyl radicals, hydrated electrons, hydrogen atoms and ·O−. The demineralization of DDBAC were quite limited with DOC reduction of 10–20% at an absorbed dose of 2.5 kGy (Fig. 4b). The DOC reduction under the acid condition was slightly lower than those under neutral and alkaline conditions.
Effect of NO3 − and HCO3 − on DDBAC radiolysis
Inorganic anions (i.e., NO3 −, HCO3 −) are always found in water and can be concentrated during the RO process. The concentrations of such anions achieve several to several dozens of mmol L−1 in the RO concentration [17]. The effects of 0.01 mol L−1 NO3 − and 0.01 mol L−1 HCO3 − on the radiolysis of DDBAC were investigated in this study (Fig. 5). The curves of the control group and the group with 0.01 mol L−1 HCO3 − were almost coincident, while the degradation of DDBAC was much slower in the presence of 0.01 mol L−1 NO3 −. The apparent kinetics constants (k) were 3.03, 2.69 and 1.45 kGy−1 for the control group and the groups with addition of 0.01 mol L−1 NO3 − and 0.01 mol L−1 HCO3 −, respectively (Table 1). Clearly slower k value was found in the group with 0.01 mol L−1 NO3 −. However, the G values in the three groups were similar, ranging 0.12–0.14 μmol J−1. The slower reaction rates were attributed to the scavenging effect of NO3 − and HCO3 − for reactive species, such as ·OH, e −aq and ·H [8]. For NO3 −, it can scavenge ·OH and e −aq , and the equations and constants were shown in Eqs. (1–3) [18, 19]. Compared to NO3 −, HCO3 − can only scavenge ·OH (Eq. (4)), and its reaction kinetic constant was much slower than that in NO3 − [8]. Therefore, DDBAC was degraded at a slower rate in the presence of NO3 − than that with HCO3 −.
Effect of 2-propanol and tert-butanol on DDBAC radiolysis
The effects of organic matters on the radiolysis of DDBAC were investigated (Fig. 6). The degradation of DDBAC was clearly slower in the presence of tert-butanol and 2-propanol. At an absorbed dose of 1.0 kGy, more than 96% of DDBAC was degraded in the control group, while the removal ratios were only 50 and 29% in the presence of tert-butanol and 2-propanol, respectively. The apparent kinetics constants (k) were 3.03, 0.75 and 0.31 kGy−1 for the control group and groups with addition of 0.01 mol L−1 tert-butanol and 2-propanol, respectively (Table 1). The G value at 1.0 kGy also decreased from 0.14 μmol J−1 in the control group to 0.04–0.07 μmol J−1 with the addition of tert-butanol and 2-propanol. The obvious decrease of DDBAC degradation kinetics and G values should be attributed to the loss of reactive species, which is consistent with the previous reports [8]. Both 2-propanol and tert-butanol efficiently scavenged ·OH, and higher rate constant was found between 2-propanol and ·OH. Additionally, 2-propanol scavenged ·H. Therefore, the degradation of DDBAC was slower with 2-propanol than that with tert-butanol. The degradation of DDBAC was attributed to the reaction with ·OH, ·H and e −aq . Based on the three rate constants (k), DDBAC degradation rate constant ratios of ·OH, ·H and e −aq can be calculated as follows.
Based on the results, the reaction with ·OH radical played the most important role in the degradation of DDBAC, followed by ·H and e −aq in sequence.
Biotoxicity changes during radiolysis of DDBAC
As a non-oxidizing biocide, DDBAC is quite toxic to organisms and humans. The acute toxicity changes during radiolysis of 10 mg L−1 DDBAC were investigated using bioluminescence inhibition test (Fig. 7). At an absorbed dose of 0.2 kGy, the concentration of DDBAC was reduced to lower than the detected limit, and the bioluminescence inhibition ratio decreased from 97.5 to 67.5%. The bioluminescence inhibition ratio kept decreasing to 40.4% at 0.5 kGy, and then kept steady with the further increase of absorbed dose. Considering only 33% of DOC was removed at an absorbed dose of 3.0 kGy, the degradation intermediates still kept around 40% of the initial toxicity of DDBAC.
Conclusions
The degradation of a non-oxidizing biocide DDBAC was investigated using gamma irradiation. The DDBAC was removed by 70–100% depending on the initial concentration and the absorbed dose, but the DOC removal ratios only ranged 10–33%. The degradation kinetics of DDBAC fitted well with the pseudo first-order reaction kinetics. With the increase of initial DDBAC concentration, the rate constants decreased, but the G value increased. With the pH values of the solution increased from 3 to 11, the rate constants had only a slight decrease, and the G values kept steady. With the addition of 0.01 mol L−1 NO3 − and HCO3 −, the rate constants decreased, but the G values kept steady. With the addition of 2-propanol and tert-butanol, both rate constants and G values had a sharp decrease. The acute toxicity of 10 mg L−1 DDBAC can be removed by 60% at absorbed doses of 0.5–3.0 kGy. The results showed that gamma irradiation was quite effective to oxide DDBAC and its toxicity, and the degradation intermediates should be further investigated in the following study.
References
Perez-Gonzalez A, Urtiaga AM, Ibanez R, Ortiz I (2012) State of the art and review on the treatment technologies of water reverse osmosis concentrates. Water Res 46(2):267–283. doi:10.1016/j.watres.2011.10.046
von Medeazza GLM (2005) “Direct” and socially-induced environmental impacts of desalination. Desalination 185(1–3):57–70. doi:10.1016/j.desal.2005.03.071
Majamaa K, Johnson JE, Bertheas U (2012) Three steps to control biofouling in reverse osmosis systems. Desalination Water Treat 42(1–3):107–116. doi:10.1080/19443994.2012.682965
Kim T-S, Park H-D (2016) Lauroyl arginate ethyl: an effective antibiofouling agent applicable for reverse osmosis processes producing potable water. J Membr Sci 507:24–33. doi:10.1016/j.memsci.2016.01.056
Tang F, Hu H-Y, Wu Q-Y, Tang X, Sun Y-X, Shi X-L, Huang J-J (2013) Effects of chemical agent injections on genotoxicity of wastewater in a microfiltration-reverse osmosis membrane process for wastewater reuse. J Hazard Mater 260:231–237. doi:10.1016/j.jhazmat.2013.05.035
Westerhoff P, Moon H, Minakata D, Crittenden J (2009) Oxidation of organics in retentates from reverse osmosis wastewater reuse facilities. Water Res 43(16):3992–3998. doi:10.1016/j.watres.2009.04.010
Benner J, Salhi E, Ternes T, von Gunten U (2008) Ozonation of reverse osmosis concentrate: kinetics and efficiency of beta blocker oxidation. Water Res 42(12):3003–3012. doi:10.1016/j.watres.2008.04.002
Guo Z, Guo A, Guo Q, Rui M, Zhao Y, Zhang H, Zhu S (2017) Decomposition of dexamethasone by gamma irradiation: kinetics, degradation mechanisms and impact on algae growth. Chem Eng J 307:722–728. doi:10.1016/j.cej.2016.08.138
Wang JL, Xu LJ (2012) Advanced oxidation processes for wastewater treatment: formation of hydroxyl radical and application. Crit Rev Environ Sci Technol 42(3):251–325. doi:10.1080/10643389.2010.507698
Wang JL, Chu LB (2016) Irradiation treatment of pharmaceutical and personal care products (PPCPs) in water and wastewater: an overview. Radiat Phys Chem 125:56–64. doi:10.1016/j.radphyschem.2016.03.012
Zheng M, Xu G, Pei J, He X, Xu P, Liu N, Wu M (2014) EB-radiolysis of carbamazepine: in pure-water with different ions and in surface water. J Radioanal Nucl Chem 302(1):139–147. doi:10.1007/s10967-014-3322-8
Solpan D, Torun M (2012) The removal of chlorinated organic herbicide in water by gamma-irradiation. J Radioanal Nucl Chem 293(1):21–38. doi:10.1007/s10967-012-1771-5
Wang L-S, Wei D-B, Wei J, Hu H-Y (2007) Screening and estimating of toxicity formation with photobacterium bioassay during chlorine disinfection of wastewater. J Hazard Mater 141(1):289–294. doi:10.1016/j.jhazmat.2006.07.001
Chu LB, Yu SQ, Wang JL (2016) Gamma radiolytic degradation of naphthalene in aqueous solution. Radiat Phys Chem 123:97–102. doi:10.1016/j.radphyschem.2016.02.029
Shah NS, Khan JA, Nawaz S, Khan HM (2014) Role of aqueous electron and hydroxyl radical in the removal of endosulfan from aqueous solution using gamma irradiation. J Hazard Mater 278:40–48. doi:10.1016/j.jhazmat.2014.05.073
Criquet J, Leitner NKV (2015) Reaction pathway of the degradation of the p-hydroxybenzoic acid by sulfate radical generated by ionizing radiations. Radiat Phys Chem 106:307–314. doi:10.1016/j.radphyschem.2014.07.016
Badruzzaman M, Oppenheimer J, Adham S, Kumar M (2009) Innovative beneficial reuse of reverse osmosis concentrate using bipolar membrane electrodialysis and electrochlorination processes. J Membr Sci 326(2):392–399. doi:10.1016/j.memsci.2008.10.018
Jo E-Y, Lee T-K, Kim Y, Park C-G (2016) Effect of anions on the removal of bisphenol A in wastewater by electro-oxidation process. Desalination and Water Treatment 57(60):29500–29508. doi:10.1080/19443994.2016.1197156
Liu Y, He X, Duan X, Fu Y, Fatta-Kassinos D, Dionysiou DD (2016) Significant role of UV and carbonate radical on the degradation of oxytetracycline in UV-AOPs: kinetics and mechanism. Water Res 95:195–204. doi:10.1016/j.watres.2016.03.011
Acknowledgements
This work was supported by Changjiang Scholars and Innovative Research Team in University [IRT-13026]; and the Science & Technology project of Tsinghua University [20141081125].
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xu, Z., Zhang, X., Huang, N. et al. Oxidation of benzalkonium chloride by gamma irradiation: kinetics and decrease in toxicity. J Radioanal Nucl Chem 312, 631–637 (2017). https://doi.org/10.1007/s10967-017-5255-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-017-5255-5