Abstract
The electron beam (EB) radiolysis characteristics of carbamazepine (CBZ) in pure-water with different ions and that in surface water were studied in this paper. It suggested that the ·OH, ·H and e −aq all played roles on CBZ EB degradation, and the ·OH played the vital role. Acidic solution was favorable for CBZ degradation, while alkaline environment inhibited it. HSO4 – and SO3 2− enhanced the CBZ degradation, but CO3 2−, NO2 −, NO3 − NH4 + and Cl− inhibited. In surface water, the EB-radiolysis was an effective way to degrade CBZ; and CBZ might evolve in three different ways during EB radiation: reduction by e −aq and ·H (intermediate 10,11-dihydrocarbamazepine (I)), oxidization by ·OH (intermediates 10,11-dihydro-10-11-expoxycarbamazepine (II) and 2(3)-hydroxycarbamazepine (III)) and hydration into 10,11-dihydro-10-hydroxycarbamazepine (IV) and finally the intermediates were all mineralized into CO2, H2O, N2 and NH4 +. All the results contribute to study the EB-radiolysis of pharmaceuticals in surface water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
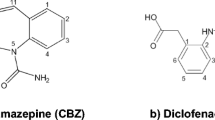
Carbamazepine (CBZ), the structure was shown in Fig. 1, a widely consumed psychotropic pharmaceutical, was one of the most commonly detected persistent pharmaceuticals in the environment [1]. It could widely impact the surface water and groundwater because of its widespread detection in wastewaters (up to 6.3 µg L−1), surface waters (up to 1.1 µg L−1), and drinking water (30 ng L−1) [2, 3]. It is refractory to both conventional and advanced wastewater treatment processes [4, 5]. In fact, CBZ led a great impact on organisms, for instance, plants causing bioaccumulation in aquatic organisms and inhibiting the growth and morphology of human embryonic cells mixed with other pharmaceuticals. In addition, it could also restrain predator avoidance of fish when it caused synergy with antidepressants [5].
Transformation of CBZ by different methods have been reported, such as biotechnology [6–9] and oxidation technique (ozonation [10], direct photolysis [11], TiO2 caused photocatalytic degradation [12], UV treatment [13] and UV/H2O2 degradation [14] ). However, the removal efficiency of CBZ was very low by biotechnology [15, 16] or abundant transformation products (TPs) (i.e. hydroxy-carbamazepone, acridine, acridone, aminobenzoic) were produced from above biochemistry treatment. Even resorting to the collaboration of them such as enhanced biodegradation of carbamazepine after UV/H2O2 advanced oxidation [5], the operations were multi-step and cumbersome. The electron beam (EB) radiation technology, one of the AOPs [17], is an efficient, safe, simple and completely mineralized method and has showed great promise during the last three decades for efficient treatment of organic pollutants, especially for the thorough decomposition of biorefractory compounds [18–23].
As shown in reaction (1), water is degraded into hydroxyl free radical (·OH), hydrogen free radical (·H), and hydrated electron (e
−aq
) with different G-values (μmol J−1) under EB irradiation. The radicals can react with the pollutants and caused its degradation [17, 20]. The ·OH has a strong oxidative ability with oxidation potential (E0 = 2.8 V), and it can efficiently oxidize the organic compounds in aqueous solutions, while e
−aq
and ·H can reduce the targeted organic and e
−aq
has a strong reductive ability (E0 = −2.9 V) [17].
The surface water contained a variety of constituents, such as ions, dissolved organic matter (DOM) and suspended solids (SS); therefore, it is necessary to study the effects of these different constituents on CBZ degradation during EB radiation. Thus, the influence of different ions, such as Na+, NH4 +, Cl−, CO3 2−, HCO3 −, SO3 2−, SO4 2−, HSO4 −, NO2 −, NO3 − on CBZ EB radiolytic degradation were investigated in this paper. Then, the degradation characteristics of CBZ in surface water were studied. Finally, a probable degradation pathway of CBZ EB-radiolysis was proposed. This study (such as the function of hydroxyl radicals) gave some assistance to the investigation on disposal of other pharmaceuticals in surface water, even photocatalysis of drugs in water, generally ·OH played a vital role in the degradation.
Materials and methods
Materials
CBZ (>98 %), methanol (HPLC grade), and acetonitrile (LCMS grade) were obtained from Sigma-Aldrich. Formic acid (HCOOH), acetic acid (CH3COOH), oxalic acid (H2C2O4), malonic acid (COOHCH2COOH), succinic acid (COOH(CH2)2COOH), NaCl, NH4Cl, Na2CO3, NaHCO3, Na2SO3, Na2SO4, NaHSO4, NaNO2, and NaNO3 were all purchased from Shanghai Chemical Reagent Co. Ltd. All chemicals were of analytical grade unless otherwise stated. The pure-water used in the experiments was prepared by filtering through a Millipore Milli-Q system (resistance >18.2 MΩ). The surface water was collected from a local river and the 0.45 μm filters were used to filter the surface water. All experiments were performed at room temperature. NaCl, NH4Cl, Na2CO3, NaHCO3, Na2SO3, Na2SO4, NaHSO4, NaNO2, and NaNO3 all were dissolved into 5 mM.
All samples were pouched in high density polyethylene (HDPE) bags. They were saturated with N2 to expel air firstly and then sealed after exhausting N2. Before the experiment we had used IC and LC/MS/MS to determine whether any intermediates were produced from HDPE bags which contained pure-water when they were irradiated under different doses (from 0.5 to 20 kGy). The results showed there were no transformation products. Therefore, HDPE could be used to pack sample solutions.
Irradiation conditions
The samples were irradiated at ambient temperatures by 1.8 MeV and variable current (0–10 mA) EB from GJ-2-II electron accelerator (Shanghai Xianfeng electrical plant, China).The samples were placed in radiation field about 30 cm away from the radiation source, and the absorbed doses were at 0.5, 1, 2, 3, 5, 10 or 20 kGy.
Analytical methods
A high performance liquid chromatography (HPLC, Agilent 1200 series), consisted of C18 column (150 × 4.6 mm) and an auto-sampler with 10 μL volume injection, was used to detect CBZ concentration at 230 nm by a VWD detector. The mobile phase was a mixture of methanol and water (55:45, v:v) at rate of 1.0 mL min−1.
Organic acids, nitrate ion (NO3 −) and nitrite ion (NO2 −) produced from CBZ EB-radiolysis were detected by ICS1100 (Dionex). A hydrophilic anion exchange column was IonPac As22 (analytical, 4 × 250 mm). The eluent was mixed with 4.5 mM Na2CO3 and 1.4 mM NaHCO3 at 1.20 mL min−1 flow rate and the injection volume was 25 uL. The suppressor was Anion Self-Regenerating Suppressor (ASRS 300 4 mm) under AutoSuppression Recycle Mode and its applied Current was 31 mA.
The other by-products of CBZ were monitored by LC/MS/MS using an Agilent 1260 LC chromatograph coupled to an Agilent 6460 mass spectrometer with electron spray ionization (ESI) interface and a heated nebulizer. A Porshell 120 100 × 3 mm EC-C18 end-capped column (2.7 μm particle size) was used, at the flow rate of 0.4 mL min−1. The injection volume was 10 μL. The mobile phase was a mixture of acetonitrile (A) and 0.1 % HCOOH in water (B); the gradient was operated from 5 to 95 % A for 8 min, from 95 to 100 % A for 2 min, held at 100 % for 2 min, and back to the initial conditions in 3.5 min. Mass spectrometry full scanning analysis was performed in the range of 50–500 m/z. The positive electron spray ionization (ESI (+)) operating conditions of the source were as follows: capillary voltage, 4000 V; nebulizer pressure, 40 psi; drying gas flow, 8 L min−1 at a temperature of 300 °C; nozzle voltage, 0 V. The negative electron spray ionization ESI (−) operating conditions of the source were as follow: capillary voltage, 3250 V; nebulizer pressure, 40 psi; drying gas flow, 7 L min−1at a temperature of 350 °C; nozzle voltage, 500 V.
Results and discussion
Effect of radical scavengers on CBZ degradation
When aqueous solution was irradiated by high-energy electrons, the main active species products, ·OH, e −aq and ·H were generated [6].
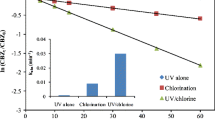
In order to investigate which active species played the leading role in the degradation of CBZ, the solution was saturated by N2, N2O and 0.317 mol/L tert-butanol with N2. As shown in Fig. 2, the higher degradation rate was achieved in N2O situated solution, the lower degradation rate happened in N2 saturated solution containing 0.317 mol/L tert-butanol. When the solution was saturated with N2O, the reaction of e −aq and ·H with N2O forms ·OH active species (reaction (2) and (3)). In N2 saturated solution,·OH, e −aq and ·H were all existed and should be considered in the reactions process [17], while in N2 saturated solution containing 0.317 mol/L tert-butanol, e −aq was the main reactive species in the CBZ solutions because in the tert-butanol solution ·OH and ·H are scavenged by tert-butanol (reaction (4) and (5)). It came to the conclusion that ·OH, ·H and e −aq played roles in the CBZ degradation process, and ·OH played the vital role.
Effect Na2SO3, Na2SO4 and NaHSO4 on CBZ degradation
As shown in Fig. 3, the addition of Na2SO3 and NaHSO4 enhanced the CBZ degradation, while Na2SO4 had no effect on the CBZ degradation. In the inset of Fig. 3, the pH value increased in Na2SO3 solution, decreased in the initial and Na2SO4 solutions and had little change in NaHSO4 solution during EB radiation.
Generally, sulfite ion (SO3 2−) quickly reacts with ·OH (\( {\text{k}}_{{{\text{SO}}_{3}^{2 - } /{ \cdot}{\text{OH}}}} = 5.5 \times 10^{9} {\text{M }}^{ - 1} {\text{s}}^{ - 1} \)) (reaction (6)); therefore, SO3 2− was recommended as a ·OH scavenger in the EB process [25]. As shown in Fig. 3, when SO3 2− was added before EB irradiation, the CBZ concentration decreased to 85.4 %. It showed that CBZ could react with SO3 2− directly before EB irradiation. During EB irradiation, CBZ degraded quickly in Na2SO3 solution and the pH value gradually rose to 10.67. Sulfite radical (·SO3 −) is produced (reaction (6)). The ·OH and ·H are decreased while the e −aq increases (reaction (7)) and the O·− occurs (reaction (8)), because of the increase of pH level. Therefore, ·SO3 −, e −aq and O·− played roles in decomposition of CBZ in Na2SO3 solution.
Recently, sulfate radical (·SO4 −) had received attention because of its high reactivity with organic pollutants such as pharmaceuticals [26–28], but the ·SO4 − almost had no effect on CBZ degradation during EB irradiation from Fig. 3.
The enhancement of degradation in NaHSO4 solution (pH 2.45 before irradiation) illustrated that the degradation of CBZ was enhanced in acidic solution. Because the e −aq can immediately react with H+ to form ·H (reaction (9)) during EB radiation; therefore, ·H played a more important role on CBZ degradation than e −aq .
Effect of Na2CO3, NaHCO3 on CBZ degradation
The experiment about EB-radiolysis of CBZ in solutions with 5 mM Na2CO3 and 5 mM NaHCO3 was performed. As shown in Fig. 4, the curves of initial and NaHCO3 almost overlapped, while the lower degradation rate happened in Na2CO3 solution.
Due to the bicarbonate ion (HCO3 −) slowly reaction with e −aq (\( {\text{k}}_{{{\text{HCO}}_{3}^{ - } /{\text{e}}_{\text{aq}}^{ - } }} = 1.0 \times 10^{6} \;{\text{M }}^{ - 1} {\text{s}}^{ - 1} \)), and ·OH (\( {\text{k}}_{{{\text{HCO}}_{3}^{-} /{\text{e}}_{\text{aq}}^{ - } }} = 8.5 \times 10^{6} \;{\text{M }}^{ - 1} {\text{s}}^{ - 1} \)) [29], HCO3 − had a bit effect on CBZ degradation [20]. However, the carbonate ion (CO3 2−) had a large suppression on CBZ degradation, as it slowly reacts with e −aq (\( {\text{k}}_{{{\text{CO}}_{3}^{{2 - { \cdot }}} /{\text{e}}_{\text{aq}}^{ - } }} = 3.9 \times 10^{5} {\text{M }}^{ - 1} {\text{s}}^{ - 1} \)), whereas quickly reacts with ·OH (reaction (10), (11)) [24]. Therefore, CO3 2− was regarded as a suitable ·OH scavenger in the EB irradiation process [30] and It was further demonstrated that ·OH was very important on CBZ degradation.
In the inset of Fig. 4, the initial pH value of NaHCO3 solution was 8.43 and the initial pH value of Na2CO3 was 11.21. Both of them had little decrease during EB radiation. The inhibition in Na2CO3 alkaline solution might be also partly due to the elimination of ·OH and ·H by OH− (reaction (12) and (13)) [24]. This result further illustrated that ·OH and ·H played an important role on CBZ degradation and the degradation rate of CBZ was inhibited in alkaline solution.
Effect of NaNO2, NaNO3 on CBZ Degradation
As shown in Fig. 5, it caused a huge depression on CBZ degradation in NaNO2 solution. It could be attributed to the decrease of ·OH, ·H and e −aq by NO2 −, as shown in reaction (14)–(17) [31] in EB-radiolysis process.
The degradation rate of CBZ in NaNO3 solution was decreased during EB radiation, because NO3 − ion acts as a scavenger of ·OH, ·H and e −aq radicals (reactions (18)–(27)) [31] which played roles in CBZ degradation rate. Although the rate constant is lower with HO· radical \( {\text{k}} = (0.88 - 1.2) \times 10^{8} \;{\text{M}}^{ - 1} {\text{s}}^{ - 1} \), NO3 − could inhibit the CBZ degradation when NO3 − concentrations was high, just as Velo et al. and Ocampo-Pérez et al. illustrated the decrease of diatrizoate and cytarabine degradation rates occurred when high NO3 −concentrations (about 5 mM) were added [31, 32].
The effect of NO2 − on the CBZ degradation constant was more marked in comparison to the NO3 −, because the rate constant is of a higher order of magnitude for the ·OH than for NO3 −, \( {\text{k}} = 6.0 \times 10^{9} \;{\text{M}}^{ - 1} {\text{s}}^{ - 1} \)
As showed the inset of Fig. 4, pH value had a little increase (to 6.95) in both NaNO2 and NaNO3 solutions. It was explained that the H+ (reaction (1)) from the irradiation of H2O is eliminated by OH− (reactions (14), (16)) in NaNO2 solution and reactions (18), (23) and (24) in NaNO3 solution.
Effect of NaCl &NH4Cl on CBZ degradation
As shown in Fig. 6, sodium ion (Na+) didn’t impact on the decrease of CBZ within 10 kGy, while chloride ion (Cl−) and ammonium ion (NH4 +) had a little inhibition on CBZ degradation. As the study of Ocampo-Pérez et al. [1] showed, Cl− can eliminate ·OH (reaction (28)) while the radical formed (ClOH·−) may again form the ·OH radical (reaction (29), and may react with e −aq or H3O+, forming the Cl· (reactions (30) and (31)), which contributed to remove H· and e −aq from the medium (reactions (32)–(35)). Therefore, the slight inhibition could be explained that the reaction with HO· is a reversible reaction and the Cl− removes H· and e −aq from the medium. Apart from the effect of Cl−, the existence of NH4 + in solution might also inhibit the degradation of CBZ because NH4 + could produce from CBZ degradation under EB irradiation which would be illustrated below.
As shown in the inset of Fig. 6, the pH values of initial, NaCl and NH4Cl had little difference.
CBZ degradation in surface water
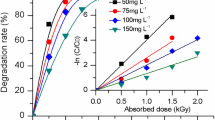
As shown in Fig. 7, more than 99 % of CBZ was removed from the surface water with or without the suspended solids (SS) at the absorbed dose of 5 kGy. However, the degradation rate of CBZ radiolysis was still lower in surface water than that in pure water. Additionally, the SS in surface water inhibited the degradation of CBZ.
Different water sources could make the results of CBZ degradation different on account of various components in water (ions, dissolved organic matters (DOM) and SS) [33].Ions, DOM and SS in surface water, which had side effects, could affect the degradation rate of CBZ. As Figs. 7, 8 showed, they all depressed the degradation of CBZ, because some ions, DOM and SS in surface water could compete with the CBZ for active radicals (·OH, e −aq and ·H during EB irradiation).This result strongly suggested that a higher absorbed dose was needed for the CBZ contaminated surface water treatment.
Radiolysis process of CBZ in surface water
The intermediates of CBZ in surface water were detected by IC and LC/MS/MS. As showed in Table 1, the determined organic acids were formic, acetic, oxalic, malonic, and succinic acid and the inorganic ions were NO2 −, NO3 − and NH4 + using IC. The concentration change of several short-chain carboxylic acids showed in Fig. 7. Malonic, and succinic acid were detected in the experiment suggested that the benzene ring was attacked and then opened. Previous study reported that the organic nitrogen might be transformed into nitrogen (N2), NH4 + and NO3 − during the oxidative degradation of CBZ [14]. Fig. 7 depicted that the concentration of NO2 − and NO3 − generated from CBZ EB degradation was very little and finally disappeared after 15 kGy and the production of NH4 + increased as the increase of EB absorbed dose. There was a difference between the calculated values and the measured values of total nitrogen (NO2 − NO3 − and NH4 +).Therefore, it was deduced that the organic nitrogen was mainly transformed to NH4 + and N2. It was consistent with the inhibition of NH4 + on CBZ EB degradation was due to the production of NH4 + from CBZ EB-radiolysis, as showed in Fig. 6.
Other complex intermediates of CBZ radiolysis were detected by LC/MS/MS, and the determined products were listed in Table 2. CBZ, occurred redox reaction with HO·, H· and e −aq during EB-radiolysis, and oxidation reaction usually acted the vital role. The olefinic double bond on the central heterocyclic ring of CBZ molecule was usually reactive [34]. On the central heterocyclic ring, CBZ could be hydrogenated to 10,11-dihydrocarbamazepine (I) by ·H [35] and hydrated to 10,11-dihydro-10-hydroxycarbamazepine (IV) by ·OH and ·H [11]. The epoxidation of CBZ to form epoxide 10,11-dihydro-10-11-expoxycarbamazepine (II) was also found in our study, which was generally mentioned in many other studies [34, 36–38]. Additionally, ·OH could attack on the two outside aromatic rings of CBZ to form 2(3)-hydroxycarbamazepine (III) [36]. As EB dose increased, the center heterocyclic and benzene rings were splintered into some short-chain acids which detected by IC and were finally mineralized into CO2, H2O, NH4 + and N2.
On the basis of the intermediates detected by LC/MS/MS and the various ions detected by IC during irradiation, a possible EB radioytic degradation of CBZ was proposed as shown in Fig. 9.
Conclusions
CBZ degradation by EB radiolysis had been demonstrated to be an effective way in water. The results showed that ·OH provided a powerful impact on CBZ degradation compared to e −aq . CBZ degradation in acidic solutions was better than that in alkaline solution except the Na2SO3 solution. SO3 2− should be avoided in surface water, because the synthesis reaction of SO3 2−and CBZ hindered the mineralization, though a positive action was on the degradation of CBZ. Similarly, NO3 − should also be precautionary in surface water, because NO3 − had a suppression on CBZ degradation at high dose (>0.5 kGy). SO4 2−, HCO3 −, NH4 +, Cl−and Na+ had a little effect on CBZ EB degradation. The degradation rate of CBZ in surface water was lower than that in pure water because of the existence of ions, DOM and SS in surface water. In addition, some intermediates of CBZ in surface water were detected using IC and LC/MS/MS and then the probable degradation pathway for the mineralization of CBZ was proposed. As result of the ubiquitous presence of inorganic salt ions, DOM and SS in surface water, all of above result gave assistance to the study of the EB-radiolysis of other pharmaceuticals in surface water.
References
Loos R, Locoro G, Comero S, Contini S, Schwesig D, Werres F, Balsaa P, Gans O, Weiss S, Blaha L, Bolchi M, Gawlik BM (2010) Pan-European survey on the occurrence of selected polar organic persistent pollutants in ground water. Water Res 44(14):4115–4126. doi:10.1016/j.watres.2010.05.032
Kosjek T, Andersen HR, Kompare B, Ledin A, Heath E (2009) Fate of carbamazepine during water treatment. Environ Sci Technol 43(16):6256–6261
Zhou H, Wu C, Huang X, Gao M, Wen X, Tsuno H, Tanaka H (2010) Occurrence of selected pharmaceuticals and caffeine in sewage treatment plants and receiving rivers in Beijing, China. Water Environ Res 82(11):2239–2248. doi:10.2175/106143010x12681059116653
Xue W, Wu C, Xiao K, Huang X, Zhou H, Tsuno H, Tanaka H (2010) Elimination and fate of selected micro-organic pollutants in a full-scale anaerobic/anoxic/aerobic process combined with membrane bioreactor for municipal wastewater reclamation. Water Res 44(20):5999–6010. doi:10.1016/j.watres.2010.07.052
Keen OS, Baik S, Linden KG, Aga DS, Love NG (2012) Enhanced biodegradation of carbamazepine after UV/H2O2 advanced oxidation. Environ Sci Technol 46(11):6222–6227. doi:10.1021/es300897u
Golan-Rozen N, Chefetz B, Ben-Ari J, Geva J, Hadar Y (2011) Transformation of the recalcitrant pharmaceutical compound carbamazepine by Pleurotus ostreatus: role of cytochrome P450 monooxygenase and manganese peroxidase. Environ Sci Technol 45(16):6800–6805. doi:10.1021/es200298t
Rodriguez-Rodriguez CE, Marco-Urrea E, Caminal G (2010) Degradation of naproxen and carbamazepine in spiked sludge by slurry and solid-phase Trametes versicolor systems. Bioresour Technol 101(7):2259–2266. doi:10.1016/j.biortech.2009.11.089
Kang SI, Kang SY, Hur HG (2008) Identification of fungal metabolites of anticonvulsant drug carbamazepine. Appl Microbiol Biotechnol 79(4):663–669. doi:10.1007/s00253-008-1459-5
Marco-Urrea E, Radjenovic J, Caminal G, Petrovic M, Vicent T, Barcelo D (2010) Oxidation of atenolol, propranolol, carbamazepine and clofibric acid by a biological Fenton-like system mediated by the white-rot fungus Trametes versicolor. Water Res 44(2):521–532. doi:10.1016/j.watres.2009.09.049
Ternes TA, Meisenheimer M, McDowell D, Sacher F, Brauch HJ, Haist-Gulde B, Preuss G, Wilme U, Zulei-Seibert N (2002) Removal of pharmaceuticals during drinking water treatment. Environ Sci Technol 36(17):3855–3863. doi:10.1021/es015757k
Chiron S, Minero C, Vione D (2006) Photodegradation processes of the antiepileptic drug carbamazepine, relevant to estuarine waters. Environ Sci Technol 40(19):5977–5983
Doll TE, Frimmel FH (2005) Removal of selected persistent organic pollutants by heterogeneous photocatalysis in water. Catal Today 101(3–4):195–202. doi:10.1016/j.cattod.2005.03.005
Kim I, Yamashita N, Tanaka H (2009) Photodegradation of pharmaceuticals and personal care products during UV and UV/H2O2 treatments. Chemosphere 77(4):518–525. doi:10.1016/j.chemosphere.2009.07.041
Vogna D, Marotta R, Andreozzi R, Napolitano A, d’Ischia M (2004) Kinetic and chemical assessment of the UV/H2O2 treatment of antiepileptic drug carbamazepine. Chemosphere 54(4):497–505. doi:10.1016/S0045-6535(03)00757-4
Hu L, Martin HM, Arce-Bulted O, Sugihara MN, Keating KA, Strathmann TI (2009) Oxidation of carbamazepine by Mn(VII) and Fe(VI): reaction kinetics and mechanism. Environ Sci Technol 43(2):509–515
Tixier C, Singer HP, Oellers S, Muller SR (2003) Occurrence and fate of carbamazepine, clofibric acid, diclofenac, ibuprofen, ketoprofen, and naproxen in surface waters. Environ Sci Technol 37(6):1061–1068. doi:10.1021/es025834r
Xu G, Bu T, Wu M, Zheng J, Liu N, Wang L (2011) Electron beam induced degradation of clopyralid in aqueous solutions. J Radioanal Nucl Chem 288(3):759–764. doi:10.1007/s10967-011-0986-1
Unob F, Hagege A, Lakkis D, Leroy M (2003) Degradation of organolead species in aqueous solutions by electron beam irradiation. Water Res 37(9):2113–2117. doi:10.1016/S0043-1354(02)00620-6
Poster DL, Chaychian M, Neta P, Huie RE, Silverman J, Al-Sheikhly M (2003) Degradation of PCBs in a marine sediment treated with ionizing and UV radiation. Environ Sci Technol 37(17):3808–3815. doi:10.1021/es030363
Kwon M, Yoon Y, Cho E, Jung Y, Lee BC, Paeng KJ, Kang JW (2012) Removal of iopromide and degradation characteristics in electron beam irradiation process. J Hazard Mater 227–228:126–134. doi:10.1016/j.jhazmat.2012.05.022
Sun W, Chen L, Tian J, Wang J, He S (2013) Degradation of a monoazo dye Alizarin Yellow GG in aqueous solutions by gamma irradiation: decolorization and biodegradability enhancement. Radiat Phys Chem 83:86–89. doi:10.1016/j.radphyschem.2012.10.014
Ghanbari F, Ghoorchi T, Shawrang P, Mansouri H, Torbati-Nejad NM (2012) Comparison of electron beam and gamma ray irradiations effects on ruminal crude protein and amino acid degradation kinetics, and in vitro digestibility of cottonseed meal. Radiat Phys Chem 81(6):672–678. doi:10.1016/j.radphyschem.2012.02.014
Taghinejad-Roudbaneh M, Ebrahimi SR, Azizi S, Shawrang P (2010) Effects of electron beam irradiation on chemical composition, antinutritional factors, ruminal degradation and in vitro protein digestibility of canola meal. Radiat Phys Chem 79(12):1264–1269. doi:10.1016/j.radphyschem.2010.07.007
Buxton GV, Greenstock CL, Helman WP, Ross AB (1988) Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (⋅OH/⋅O−) in aqueous solution. J Phys Chem Ref Data 17:513–886
Shin HS, Kim YR, Ponomarev AV (2001) Influence of sulfite on radiolytic conversion of nitrate and nitrite in dilute aqueous solutions. Mendeleev Commun 1:21–23
Guan Y-H, Ma J, Li X-C, Fang J-Y, Chen L-W (2011) Influence of pH on the formation of sulfate and hydroxyl radicals in the UV/Peroxymonosulfate system. Environ Sci Technol 45(21):9308–9314. doi:10.1021/es2017363
Nfodzo P, Choi H (2011) Sulfate radicals destroy pharmaceuticals and personal care products. Environ Eng Sci 28(8):605–609. doi:10.1089/ees.2011.0045
Roshani B, Leitner NK (2011) Effect of persulfate on the oxidation of benzotriazole and humic acid by e-beam irradiation. J Hazard Mater 190(1–3):403–408. doi:10.1016/j.jhazmat.2011.03.059
Mak FT, Zele SR, Cooper WJ, Kurucz CN, Waite TD, Nickelsen MG (1997) Kinetic modeling of carbon tetrachloride, chloroform and methylene chloride removal from aqueous solution using the electron beam process. Water Res 31(2):219–228. doi:10.1016/s0043-1354(96)00264-3
Cooper WJ, Nickelsen MG, Green RV, Mezyk SP (2002) The removal of naphthalene from aqueous solutions using high-energy electron beam irradiation. Radiat Phys Chem 65(4–5):571–577. doi:10.1016/s0969-806x(02)00363-8
Ocampo-Pérez R, Rivera-Utrilla J, Sánchez-Polo M, López-Peñalver J, Leyva-Ramos R (2011) Degradation of antineoplastic cytarabine in aqueous solution by gamma radiation. Chem Eng J 174(1):1–8
Velo Gala I, López Peñalver JJ, Sánchez Polo M, Rivera Utrilla J (2013) Degradation of X-ray contrast media diatrizoate in different water matrices by gamma irradiation. J Chem Technol Biotechnol 88(7):1336–1343
Xu G, Liu N, Wu MH, Bu TT, Zheng M (2013) The photodegradation of clopyralid in aqueous solutions: effects of light sources and water constituents. Ind Eng Chem Res 52(29):9770–9774. doi:10.1021/ie302844v
Li J, Dodgen L, Ye Q, Gan J (2013) Degradation kinetics and metabolites of carbamazepine in soil. Environ Sci Technol 47(8):3678–3684. doi:10.1021/es304944c
Miao X-S, Metcalfe CD (2003) Determination of carbamazepine and its metabolites in aqueous samples using liquid chromatography − electrospray tandem mass spectrometry. Anal Chem 75(15):3731–3738. doi:10.1021/ac030082k
Miao XS, Yang JJ, Metcalfe CD (2005) Carbamazepine and its metabolites in wastewater and in biosolids in a municipal wastewater treatment plant. Environ Sci Technol 39(19):7469–7475
Lam MW, Mabury SA (2005) Photodegradation of the pharmaceuticals atorvastatin, carbamazepine, levofloxacin, and sulfamethoxazole in natural waters. Aquat Sci 67(2):177–188. doi:10.1007/s00027-004-0768-8
Breton H, Cociglio M, Bressolle F, Peyriere H, Blayac JP, Hillaire-Buys D (2005) Liquid chromatography-electrospray mass spectrometry determination of carbamazepine, oxcarbazepine and eight of their metabolites in human plasma. J Chromatogr, B: Analyt Technol Biomed Life Sci 828(1–2):80–90. doi:10.1016/j.jchromb.2005.09.019
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos. 11175112, 11025526, 41173120, 11305099 and 41273141).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zheng, M., Xu, G., Pei, J. et al. EB-radiolysis of carbamazepine: in pure-water with different ions and in surface water. J Radioanal Nucl Chem 302, 139–147 (2014). https://doi.org/10.1007/s10967-014-3322-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3322-8