Abstract
69mZn was produced and separated for medical applications. Possibilities and perspectives for production of radiopharmaceuticals based on 69mZn containing derivatives of thiazine, thiazoline and thiourea are considered. Each one of the latters is a zinc chelator and a nitric oxide synthase (NOS) effector at the same time. Cytotoxic effect of NOS activator and NOS inhibitors are shown in experiments with HL-60, K-562 and MOLT-4 cell lines and in bone marrow cells of the acute B-lymphoblastic leukemia patients. Some of those compounds are worthy to get selected for further application as radiopharmaceuticals including their antitumor speciements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the trends in the modern drug development research is the use of preparations with multi-faceted effects (or platforms-transporters carrying several different drugs) as well as the approved drugs application and previously untreatable clinical cases [1]. The common design of radiopharmaceuticals includes radionuclide that is chemically linked to the molecule responsible for target delivery. Such vector molecule usually does not demonstrate the drug properties but enable chelation of the radionuclide enable its delivery to tumor cells. However alternative approach is to use multicomponent systems (drug platform) that may combine several drug molecules that are linked with specific vector molecule. In some cases this enable to prolong drug properties and use multiple attack on the tumor with simultaneous protection of the healthy tissues, e.g. by the use of fullerene derivatives [2] that may carry several drugs (including metal ion isotope) and specific vector. For example, hydroxylated metallo-fullerene that contain Gd-atom demonstrate immune and antitumor activity, down-regulate more than ten angiogenic factors at the mRNA level and at the same time act as an antioxidant [3, 4].
Our approach to radiopharmaceuticals molecular design means a selection and a further chemical link between the below listed component types: 1—radionuclide that can be used as a diagnostic or therapeutic agent; 2—active organic molecule that acts as the drug itself and metal radionuclide chelator; and 3—transporter (vector) which can carry entire construction to the biological target, that may have an affinity towards the radioactive isotope. In case of metal cation radionuclide it serves as a drug substance and a linker between the vector and an active biomolecule that also serves as a multiple drug substance (Fig. 1).
69mZn isotope has a half-life of 13.78 h and the gamma decay energy of 438.6 keV [5]. This allows to consider it as a possible component of radiopharmaceuticals for both diagnostic and therapeutic purposes. Energy line of 438.6 keV can be used for SPECT diagnostics and β-radiation of daughter 69Zn isotope with E max = 906 keV makes it a therapy reliable product. Several methods for producing the 69mZn isotope were described in 1970s [5–8].
Zinc is a unique trace element responsible for a total control over the conformational patterns in some major enzymes and supramolecular biostructures (like zinc fingers-DNA, NOS dimers etc.) functioning within the body signaling pathways [9–11]. Zinc have rich coordination geometry (tetrahedral, pyramidal and octahedral) with coordination number equals 4 to 6 depending on the ligand type [13] and it can form ternary complexes as well [14, 15].
Aliphatic and heterocyclic sulfur- and nitrogen-containing radioprotectors used for their direct purpose and as the radiotherapy compensating agents may have a great future in nuclear medicine. Noteworthy, these compounds sometimes possess inhibitory properties for signaling molecules [for example, NO-synthase (NOS)] which may result in antitumor activity. The role of NO and NOS expression in the development and treatment of cancer has been widely discussed [16–18]. Besides, some recent reviews contain the statement about the participation of NO and NOS inhibitors in the mechanisms of emergence and treatment of different diseases [19–22]. In malignant bone marrow and blood cells, the increased NOS expression has been found [23]. The most prominent contribution to this increased expression level was made by inducible NOS (iNOS), while in a smaller degree—by epithelial (eNOS) and neuronal (nNOS). Figure 2 shows the NO participation in carcinogenesis. It is believed that the enabling enhancing effects of NO at the initial stage of hematopoiesis may lead to formation of stem cancer cells [24, 25]. Patients diagnosed with acute myeloid leukemia (AML), with different types of lymphoma and with some other cancers showed overexpression of iNOS [16]. Laminar hemodynamic shock which can activate a NF-κB (an element of cancerogenesis) caused the increased enzymatic production of NO via the eNOS activation [26]. At present, both NOS inhibitors and NO-donors are considered as the possible antitumor drugs [17]. Here we focus on NOS effectors (in particular, iNOS inhibitors) as the potential antitumor agents capable to be the base and the linker for radiopharmaceuticals (RP). All of these compounds are active chelators for metal ions—particularly, zinc and copper ions [12, 27].
Drug delivery process is not considered here. Delivery of zinc-containing drugs to the organs and tissues can be performed by the body’s own systems (albumine, metallothionein etc.) as well as by binding the zinc-containing drugs with nanoparticles and with specific physiological Zn-transporters (of ZIP and ZnT families) that is a great advantage of this element and its isotopes.
Experimental
Production of 69mZn radioactive isotope
69mZn (T1/2 = 13.78 h) was produced by reaction of 71Ga (γ, np) 69mZn from metallic gallium by bremsstrahlung photon beam with energy up to 55 MeV on a race-track microtron of Skobeltsyn institute of nuclear physics of MSU (Fig. 3). The yield was 0.13 MBq mA−1 h−1.
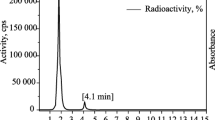
69mZn was separated in two-step process: the initial isolation of zinc from the bulk gallium target was carried out by the liquid–liquid extraction (twice) with methyl isobutyl ketone followed by an ion exchange using Dowex 1 × 8 (2 mol L−1 HCl). All processes were monitored using a gamma spectrometer (Ge-detector GR 3818 Canberra Ind., USA). The criterion for evaluation was a long-life 67Ga radionuclide. After two extraction steps more than 80 % of gallium was separated which comes from the ratio of peaks the related to isotopes 67Ga and 69mZn. The result of ion exchange chromatography is shown on Fig. 4. Yield of the carrier free 69mZn was 96 %.
NOS-effectors: active molecules for drug design
In this work, the following compounds were synthesized and used: 2-amino-5,6-dihydro-4H-1,3-thiazine hydrobromide (T1); 2-dodecylamino-5,6-dihydro-4H-1,3-thiazine hydrobromide (T2); 2-amino-5,6-dihydro-4H-1,3-thiazine salicylate (T3); 2-(2-fluorophenyl) amino-5,6-dihydro-4H-1,3 thiazine hydrobromide (T4); N-(5,6-dihydro-4H-1,3-thiazin-2-yl)-benzamide hydrobromide (T5); 2-amino-5-methyl-2-thiazoline hydrobromide (TZ6); 2-amino-5-hydroxymethyl-2-thiazoline hydrobromide (TZ7); N-(4-isopropyl-phenyl)-N-(1–iminoethyl-piperidin)-1-carbo-thioamide hydrobromide (TM8) and-N-(4-methylphenyl)-N-(1-iminoethyl) pyrrolidine-1-carbo-thioamide hydrobromide (TM9) and 1-(1-iminoethyl)-1-(4-isopropylphenyl)-3,3-dimethyl-thiourea hydrobromide (TM10) (Figs. 5, 6). The methods for synthesis of species were described earlier [28–32]. The composition and structure of the specimens were controlled by element analysis and by 1H and 13C NMR. The selection of drugs was based on various types of the NOS-inhibitory activity [33] to create the following chain of NOS effectors: NOS activator—an inert preparation (with respect to iNOS)—NOS inhibitors with an increased degree of inhibition in vivo.
Thiazine derivatives: T1—2-amino-5,6-dihydro-4H-1,3-thiazine hydrobromide; T2—2-dodecylamino-5,6-dihydro-4H-1,3-thiazine hydrobromide; T3—2-amino-5,6-dihydro-4H-1,3-thiazine salicylate; T4—2-(2-fluorophenyl)-amino-5,6,-dihydro-4H-1,3,-thiazine hydrobromide; T5—N-(5,6-dihydro-4H-1,3-thiazine-2-yl)-benzamide hydrobromide. Thiazoline (TZ) derivative: TZ6—2-amino-5-methyl-2-thiazoline hydrobromide
Thiazoline (TZ) and thiourea (TM) derivatives: TZ7—2-amino-5-hydroxymethyl-2-thiazoline hydrobromide; TM8—N-(4-isopropylphenyl)-N-(1-iminoethyl-piperidine)-1-carbothioamide hydrobromide; TM9—N-(4-methylphenyl)-N-(1-iminoethyl)-pyrrolidine-1-carbothioamide hydrobromide; TM10—1-(1-iminoethyl)-1-(4-isopropylphenyl)-3,3-dimethylthiourea hydrobromide
Preparation of cell material
Cell lines cultured in a standard way were used: HL-60 (human promyelocytic leukemia line), K-562 (chronic myeloid leukemia line) and MOLT-4 (human cell line, an acute T-lymphoblastic leukemia. The patient bone marrow samples were aspirated (3–5 mL) with diagnostic puncture of the front or rear iliac spines before chemotherapy when diagnosed with acute B-lymphoblastic leukemia (B-ALL). Blood samples from healthy donors and preparation of cell material were carried out as described previously [34]. The content of blast cells in the peripheral blood mononuclear fraction was >80 %. Lymphocytes of healthy donors of the same age group were used as control.
MTT-method is based on determining the viability of cell cultures. Living cells can recover the soluble yellow 3-(4,5-dimethylthiazole-2-yl)-2,5-tetrazolium bromide (MTT) by mitochondrial and cytoplasmic dehydrogenases to form purplish-blue formazan crystals, soluble in DMSO or isopropanol [35, 36]. The amount of formazan was determined by spectrophotometry (Microplate Reader, model 550, Bio-Rad) at λ = 550 nm. The methodology of the MTT assay was described in detail in [34], n ≥ 10 for each case. The contribution of blast cells was more than 80 %. Results were processed by the Mann–Whiney U-test (p < 0.05). The LC50 value was evaluated by the median and the t-student statistic.
Stability of compounds represented on Figs. 4 and 5 was determined spectrophotometrically in “UV PD303UV” (Apel, Japany) keeping for 2–6 days at 37 °C in the saline solution. Stability of Zn (69mZn)-complexes with T5 and TM8 was determined similarly in the alcoholic solutions for 3 days.
In vitro tests The literature data on the in vitro experiments were taken for comparison from previous studies [31, 32, 37] which were carried out using the liquid scintillation counting method with [3H-l-arginine]. iNOS in these studies were isolated from mouse macrophages stimulated with LPS ((lipopolysaccharides, ≪Cayman Chemical≫, USA). The catalytic activity of enzyme was determined by the rate of accumulation of [3 H-l-citrulline].
In vivo tests The NOS-inhibitory activity of compounds were carried out by EPR spectroscopy [38] with spin trap (Fe2+-diethyl-dithiocarbamate complex) on the Swiss line white inbred male mice of the Swiss line, aged 5 months, weighing 27–30 g. LPS from E. coli (a dose of 1.5 mg kg−1 (0.5 mL of the saline)) were used.
Complexes of zinc with above compounds were obtained like in [39] by addition of ZnCl2 in the alcoholic solution of specimen (the ratio 2:1) under stirring and with further precipitation. The composition of complexes was checked by element analysis and 1H-NMR. To obtain the 69mZn-T5 and 69mZn-TM8 labeled compounds, both T5 and TM8 alkaline forms were applied. The latters were slowly dissolved in an appropriate organic media followed by addition of strictly necessary amount of the 69mZnCl2 solution. White crystalline precipitate of complex salt has been appeared instantly.
As it comes to a pure 69mZnCl2, the water solution of carrier free 69mZn was evaporated with a subsequent addition of the enough-minimal volumes of ZnCl2 dissolved in the very same organic media as the active compounds were dissolved in. To evaluate the labeled compounds stability, gamma-spectrometry, UV-spectrophotometry and radioTLC were employed (98 and 95 % radiochemical purity for 69mZn-T5 (A) and 69mZn-TM8 (B) complexes, respectively). The systems: methanol:H2O (95:5 %) for (A) and BAM (butanol-acetone-formic acid) (1:1:1) for (B) were used in radioTLC, demonstrating the values of R f = 0.65 and 0.80, respectively.
Results and discussion
Active molecules
Interconnection between the NOS-inhibitory activity of administered compounds and the cell survival patterns were described in [33]. In healthy donor cells, the reduction of NO level (at the increasing NOS inhibitory activity) leads to a sharp (trigger) change of the impact mechanism on the system whose behavior after the jump to a new higher level of survival does not depend on the concentration of NO (within the margin error). This reminds the buffer system properties in terms of its capability to demonstrate the “jump” of cell viability with a following stabilization of the higher level. For leukemic cells, such a jump is unseen except for the K-562 cell line.
The highest value of the “therapeutic index” as TI = LC50 (healthy donors)/LC50 (leukemic cells) is expected to be observed for compounds with NOS-inhibitory level within the region right after the “jump”. The values of the TI for our compounds are listed in Table 1, where the magnitudes of LC50 were obtained by MTT-test method. If we consider the compounds as drugs for therapy, it is evident that the compounds from part II (Table 1) have no practical interest. Even though TM8 (NOS-activator) and T5 compounds were not selective towards any particular cancer cell type, they both show a clear down-regulation in all leukemic cells tested. Unlike TM8, a T5 compound has a low toxic effect on healthy cells and, besides, it possesses the antihypotensive (antishock) activity [40]. In addition to its anticancer properties, it may be of interest. A TM10 (TI = 10) compound seems promising for treatment of B-ALL, while a TZ7 (TI = 20) compound demonstrates a selective activity against HL-60. A high value of TI is observed for TM10 (TI = 10). This is a radiosensitizer with a dose modification factor (DMF) ~0.8.
The replacement of hydrobromide on salicylate as a counter ion (compound T3) showed a slight increase in cytotoxicity that correlates with the literature data on the antitumor activity of salicylates and acetylsalicylate [41, 42]. However, these properties of salicylates require further study.
Preliminary studies of 69mZn-T5 effect on MOLT-4 cell line showed a slight decrease in healthy cells survival compared to leukemic cells and about 3-fold increase of TI. However, this work requires a special long-term study, which is planned in the future.
Stability of compounds in saline solution
T1, T3, TM8, T5, TZ6 and TZ7 compounds showed high stability and a lack of significant hydrolysis under the experimental conditions. T2, a thiazin derivative, with long hydrophobic “tail”, demonstrates a high cytotoxicity and a firm tendency to aggregation and a formation of vanable nanoparticles. This leads to changes in a spectrophotometric patterns and promotes a peak shift at λ = 220 nm. The T4 compound (dihydrothiazin derivative) underwent hydrolysis with partial formation of thiazine and additional intermediates during long storage (more than 6 days). The latter were not analyzed in this paper. No significant correlation was observed between the cell survival and compounds stability. The spectrophotometric data showed no significant changes in the stability of the complexes 69mZn-T5 and 69mZn-TM8 during time suitable for an active work with this isotope (3 days).
Zinc isotopes in the NOS-effectors based drugs
All compounds represented above exhibit anticancer activity and possesses the nitrogen, oxygen and sulfur in their structures. This makes them capable to form stable chelates [12] with a variety of zinc (or other metal) isotopes. Once incorporated into the drug-vector containing complex, Zn isotopes may provide a benefit of the origin and a further development of a new radiofarmaceutical family. 69mZn is one of these isotopes, combining magnetic and radiochemical properties and representing a promising object for the creation of new radiopharmaceuticals. This can be made, in particular, on the basis of NOS-effectors. Radiochemical purity of 69mZn obtained is seen in Fig. 3b (line of 438.6 keV).
In this way, we have two perspective components for radiopharmaceutical: the most promising compounds T5 and TM8—and radioactive 69mZn isotope with suitable parameters. The model experiments including the introduction of 69mZn isotope (T 1/2 = 13.78 h, A ~1.5 105 Bq mg−1) into compounds did not lead to significant changes in their stability (determined by spectrophotometry) at least for 3 days (data not shown). That means a very low level of radiolysis. However, this requires additional verification.
Conclusions
The next steps of research should be the careful investigation of 69mZn-complexes, the carrier screening and the binding of zinc-labeled compounds with a carrier suitable to manage an organ specific targeted delivery of the new pharmaceutical agent.
References
De la Iglesia S, Lypez-Jorge CE, Gymez-Casares MT, Castellano AL, Cabrera PM, Brito JL, Cabrera AS, Labarta TM (2009) Induction of apoptosis in leukemic cell lines treated with captopril, trandolapril and losartan: a new role in the treatment of leukemia for these agents. Leuk Res 33:810–816
Orlova MA, Trofimova TP, Shatalov OA (2013) Fullerene nanoparticles operating the apoptosis and cell proliferation processes in normal and malignant cells. Der Pharmacia Lett 5:99–139
Wang J, Chen C, Li B (2006) Antioxidative function and biodistribution of [Gd@C82(OH)22] n nanoparticles in tumor-bearing mice. Biochem Pharmacol 71:872–881
Chen C, Xing G, Wang J (2005) Multihydroxylated [Gd@C82(OH)22] n nanoparticles: antineoplastic activity of high efficiency and low toxicity. Nano Lett 5:2050–2057
Pu Z, Yang J, Kong X (2003) Cross-section measurements for (n, 2n), (n, p) and (n, n′α) reactions on gallium isotopes in the neutron energy range of 13.5–14.6 MeV. Appl Radiat Isotop 58:723–726
Yagi M, Kondo K (1977) Preparation of carrier-free Zn-69 m by the 71 Ga(γ, pn) reaction. Radiochem Radioanal Lett 30:173–178
Demichelis F, Guidetti M, Miraldi E, Oldano C (1968) Isomeric cross-section ratio for reactions producing the isomeric pairs Zn-69 and Zn-69m, Zn-71 and Zn-71m. Il Nuovo Cimento 58:177–190
Cohen IM, Guevara SR, Arribere MA, Iljadica MCF, Kestelman AJ, Ohaco RA, Segovia MS, Yunes AN (2005) Determination of nuclear constant of reactions induced on zinc by short irradiations with the epithermal and fast component of reactor neutron spectrum. Radiochim Acta 93:543–546
Maret W (2012) New perpectives of zinc coordination environments in proteins. J Inorg Biochem 111:110–116
Maret W (2011) Metals on the move: zinc ions in cellular regulation and in the coordination dynamics of zinc proteins. Biometals 24:411–418
Orlova MA, Orlov AP (2011) Role of zinc in an organism and its influence on processes leading to apoptosis. Br J Med Med Res 1:239–305
Lee Y, Lin Y, Lima C (2014) Factors controlling the role of zn and reactivity of zn-bound cysteines in proteins: application to drug target discovery. J Chin Chem Soc 61:142–150
Dudev T, Lim CJ (2003) Metal binding and selectivity in zinc proteins. Chin Chem Soc 50:1093–1102
Jacob C, Maret W, Vallee BL (1998) Control of zinc transfer between thionein, metallothionein and zinc proteins. Proc Natl Acad Sci USA 95:3489–3494
Auld DS (2009) The ins and outs of biological zinc sites. Biometals 22:141–148
Brandao MM, Soares E, Salles TSI, Saad STO (2000) Expression of inducible nitric oxide synthase is increased in acute myeloid leukemia. Acta Haematol 106:95–99
Li CQ, Wogan GN (2005) Nitric oxide as a modulator of apoptosis. Cancer Lett 226:1–15
Sawa T, Arimoto H, Akaike T (2010) Regulation of redox signaling involving chemical conjugation of protein thiols by nitric oxide and electrophiles. Bioconjug Chem 21:1121–1135
Rapoport RM (2014) Nitric oxide synthase inhibition and endothelian-1-dependent arterial pressure elevation. Front Pharmacol 5(57):1–8
Mukherjee P, Cinelli MA, Kang S, Silverman RB (2014) Development of nitric oxide synthase inhibitors for neurodegeneration and neuropathic pain. Chem Soc Rev 43:6814–6838
Tousoulis D, Simopoulou C, Papageorgiou N, Oikonomou E, Hatzis G, Siasos G, Tsiamis E, Stefanadis C (2014) Endothelian dysfunction in conduit arteries and in microcirculation. Novek therautic approaches. Pharmacol Ther 144:253–267
Tsutsui M, Tanimoto A, Tamura M, Mukae H, Yanagihara N, Shimokawa H, Otsuji Y (2015) Significance of nitric oxide synthases: lessons from triple nitric oxide synthases null mice. J Pharmacol Sci 127:42–52
Wallerath T, Gath I, Aulizky WE, Wallerath T, Gath I, Aulitzky WE (1997) Identification of the synthase isoforms expressed in human neutrophil granulocetes, megakaryocetes and platelets. Thromb Haemost 77:163–167
Baker M (2012) Cancer stem cells tracked. Nature 488:13–14
Tan BT, Park CY, Weissman IL (2006) The cancer stem cell hypothesis: a work in progress. Lab Invest 86:1203–1207
Cai H, McNally JS, Weber M, Harrison DG (2004) Oscillatory shear stress upregulation of endothelial nitric oxide synthase requires intracellular hydrogen peroxide and CaMKII. J Mol Cell Cardiol 37:121–125
Torres-Garcia P, Vinuelas-Zahinos E, Luna-Giles F, Espino J, Barros-Garcia FJ (2011) Zinc(II) complexes with novel 1,3-thiazine/pyrazole derivative ligands: synthesis, structural characterization and effect of coordination on the phagocytic activity of human neutrophils. Polyhedron 30:2627–2636
Proshin AN, Trofimova TP, Bachurin SO (2011) New tetrasubstituted thioureas containing the 1-iminoethyl moiety. Russ Chem Bull 60:2432–2436
Schoberl A, Magosch KH (1970) Acylierung und alkylierung von 2-amino-penthiazolin. Liebigs Ann Chem 742:74–84
Trofimova TP, Zefirova ON, Mandrugin AA, Fedoseev VM, Peregud DI, Onufriev MN, Gulyaeva NV, Proskuryakov SY (2008) Synthesis and study of NOS-inhibiting activity of 2-N-acylamino-5,6-dihydro-4H-1,3-thiazine. Mosc Univ Chem Bull 63:274–277
Trofimova TP, Pushin AN, Proshin AN, Stash AI, Mandrugin AA, Fedoseev VM, Proskuryakov SY (2007) Synthesis of new 2-amino-5-hydroxymethyl-2-thiazolines. Chem Heterocycl Comp 43:370–376
Levtsova AA, Chupakhin VI, Proshin AN, Pushin AN, Trofimova TP, Zefirova ON (2007) Design of potential NO-synthase inhibitors on the basis of 2-amino-5,6-dihydro-4H-1,3-thiazine derivatives. Mosc Univ Chem Bull 62:243–245
Orlova MA, Trofimova TP, Nikulin SV, Orlov AP (2016) Relationship between NO-synthase inhibitory activity of N-, S-containing heterocycles with their radioprotective and antileukemic properties. Vestn Mosk Univ. Ser. Khimiya. 57:269–275 (Trans.: Mosc Univ Chem Bull. 71: In Press)
Orlova MA, Osipova EYu, Roumiantsev SA (2012) Effect of 67Zn-nanoparticles on leukemic cells and normal lymphocytes. Br J Med Med Res 2:21–30
Veerman AJP, Pieters R (1990) Drug sensitivity assays in leukemia and lymphoma. Br J Haematol 74:381–383
Kaspers GJL, Pieters R, Twentyman PR, Wiesenthal LM, Veerman AJP (1993) Drug resistance in leukemia and lymphoma I. Harwood Academic Publishers, Chur
Khokhlova TD, Mandrugin AA, Trofimova TP, Fedoseev VM (2010) Adsorption of NO synthase inhibitor on dehydroxylated silica. Protect Met Phys Chem Surf 46:427–429
Stone JR, Sands RH, Dunham WR, Marletta MA (1995) Electron paramagnetic resonance evidence for the formation of a pentacoordinate nitrosyl-heme complex on soluble guanylate cyclase. Biochem Biophys Res Commun 207:575–577
Lin ZD, Zeng W (2007) Bis(2-aminopyrimidine-N1)dichloridozinc(II). Acta Crystallogr E 63:m1597
Filimonova MV, Shevchenko LI, Trofimova TP, Makarchuk VM, Shevchuk AS, Lushnikova GA (2014) About mechanism of radioprotective action of NO-synthases inhibitors. Rad Biol Radioecol (Russ) 54:500–506
Rana K, Reinhart-King CA, King MR (2012) Inducing apoptosis in rolling cancer cells: a combined therapy with aspirin and immobilized TRAIL and E-selectin. Mol Pharm 9:2219–2227
Morgan G (2005) Could vitamin S (salicylate) protect against childhood cancer? Med Hypotheses 64:661–664
Acknowledgments
This study was supported by grant of RFBR 16-08-00139.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Orlova, M.A., Trofimova, T.P., Aliev, R.A. et al. 69mZn-containing radiopharmaceuticals: a novel approach to molecular design. J Radioanal Nucl Chem 311, 1177–1183 (2017). https://doi.org/10.1007/s10967-016-5076-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-016-5076-y