Abstract
The magnetic hydrothermal cross-linking chitosan (HCC–Fe3O4) was prepared by hydrothermal carbonization and was characterized by FT-IR, X-ray diffraction and scanning electron microscopy. The as-prepared HCC–Fe3O4 was used as adsorbent to remove uranium(VI) as functions of contact time, pH, temperature and initial uranium(VI) concentration batch wise. The results indicated that the magnetic HCC–Fe3O4 was favorate for solid–liquid separation and the maximum uranium(VI) adsorption capacity was 263.1 mg/g at pH 7.0 and 25 °C. The adsorption isotherm of uranium(VI) was well fitted by the Langmuir model. The adsorption is a chemical reaction in nature proved by the well described with pseudo-second-order model. The obtained thermodynamics parameters of positive ΔH, positive ΔS, and negative ΔG denoted the adsorption was an endothermic, disorder increasement, and spontaneous process. These results demonstrated that HCC–Fe3O4 was a promising adsorbent for the enrichment of uranium(VI) from aqueous solutions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Uranium(VI) of natural activity commonly occurs in the oxide state as uranium dioxide in ore, which transforms into uranyl in the acid leach process. Inevitably, uranyl can escape into the environment in the processes including uranium ore mining, uranium purification and transformation [1, 2]. The uranyl ion is linear and has great potential to complex organic ligands, resulting in water solubility resultants [3, 4]. These products easily gets into human ecosystem owning to unique mobility, causing irreversible damage by radioactive and chemical toxicity [5, 6]. For this sake, uranium separation from uranium containing wastewater is of great importance.
Chitosan composed of (1,4)-2-amino-2-deoxy-b-d-glucan is a natural linear polysaccharide and can be fabricated by partial chitin deacetylation [7]. Due to easy functionalization with amino and hydroxyl functional groups, low toxicity, biocompatibility, and biodegradability [8], chitosan has great potential in the separation process [9]. By using attapulgite as matrix, chitosan as functional monomer, Shi et al. perpared Cu(II) ion imprinting polymer, which had good selectivity for Cu(II) over competitive metal ions. The selectivity coefficient of Cu(II)/Pb(II) and Cu(II)/Cd(II) reached 78.45 and 82.44, respectively [10]. Zeng et al. [11] prepared xanthated crosslinked chitosan resin, which had ability to adsorb Au(III) ions.Graphene oxide modified with cross-linked chitosan could adsorb Cu(II) with a maximum adsorption capacity of 202.5 mg/g [12]. However solid–liquid separation is laborious after adsorption using chitosan because of its powder formation.
Magnetization is a powerful technique to solve this problem. Magnetic iron oxide nanoparticles have attracted much attention in the area of targeted drug delivery [13], magnetic resonance imaging, catalysis [14], and environmental remediation [15]. The magnetic separation technique has shown to be a promising method for solid–liquid phase separation [16, 17].
This work aims at preparing a low-cost and highly efficient magnetic composite adsorbent with high adsorption capacity. The magnetic hydrothermal cross-linking chitosan (HCC–Fe3O4) was synthesized. The prepared materials were characterized by X-ray diffraction (XRD), fourier transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM). The adsorption of HCC–Fe3O4 toward uranium(VI) as functions of contact time, pH, temperature and initial uranium(VI) concentration were investigated. The adsorption kinetics and the possible uranium(VI) removal mechanism (Scheme 1) of HCC–Fe3O4 as well as the recovery of used HCC–Fe3O4 were investigated in detail. The results demonstrated that HCC–Fe3O4 has good adsorption performance for uranium(VI).
Experimental
Materials
Chitosan of 40 mesh size and 90 % degree of deacetylation were purchased from Shanghai Medicine Company. A stock solution of uranium(VI) (1000 mg/L) was prepared by dissolving U3O8 in a mixture solution of HCl, H2O2 and HNO3. FeCl3·6H2O, FeCl2·4H2O and arsenazo III were purchased from Shanghai Medicine Company. All other reagents used in this study were analytical grade.
Instruments
SEM images were recorded with a JEOL JSM-5900 microscope with 15 kV. XRD patterns were obtained by a Bruker D8 Advance X-Ray diffractometer with Cu–Ka source (l = 1.54178 Å). FT-IR were recorded on a Nicolet Magana IR 380 spectrometer.
Preparation and characterization of magnetic hydrothermal cross-linking chitosan (HCC–Fe3O4)
In the atmosphere of argon, acetic solution (80 mL, 5 %) were added into a three-neck rounded bottom flask, followed by addition of FeCl2·4H2O (0.0497 g) and FeCl3·6H2O (0.2027 g), the mixture was stirred for 10 min, to which 1.5 g chitosan and ammonia solution (80 mL, 5 %) were added. The mixed solution was stirred vigorously for 30 min, transferred into a 100 mL Teflon-lined stainless steel autoclave, heated at 180 °C for 12 h, cooled to room temperature, and washed three times with deionized water. After dried under vacuum, the obtained product was characterized by XRD, FT-IR, SEM.
Adsorption experiments
The effects of pH, contact time, initial uranium(VI) concentration and temperature on adsorption of uranium(VI) were investigated. The adsorption of uranium on HCC and HCC–Fe3O4 composites was conducted batch wise. For each experiment, 0.05 g of HCC or HCC–Fe3O4 composites was mixed with 100 mL of uranium solution in a conical flask. The pH value of solution was adjusted with 0.1 M HCl and NaOH solution. The conical flask was sealed and kept agitating in a shaking bath for a desired time. Then stock suspensions of HCC and HCC–Fe3O4 solution were equilibrated and then solid phase was separated by a permanent magnet and some of clear liquid was taken with a liquid transferring gun. The quantitative analysis of uranium(VI) ion was done by spectrophotometry using uranium(VI) arsenazo III complex at a wavelength 670 nm [18]. The amount of uranium(VI) adsorbed on the surface of HCC or HCC–Fe3O4 (q e ) were calculated using Eq. (1) and distribution coefficient (K d ) was counted by Eq. (2).
where C 0 (mg L−1) and C e (mg L−1) are the liquid-phase concentration of uranium(VI) at initial and equilibrium, respectively; m (g) is the absorbent mass of HCC or HCC–Fe3O4; V (L) is the suspension volume and q e (mg g−1) is the amount of uranium(VI) adsorbed on HCC or HCC–Fe3O4.
Results and discussion
Characterization
The microstructures of HCC and HCC–Fe3O4 composites were characterized by SEM, FT-IR and XRD.
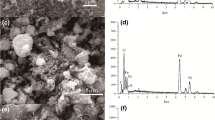
The typical SEM images of HCC and HCC–Fe3O4 are shown in Fig. 1. It could be observed from Fig. 1a that HCC showed a disordered fiber structure while the surface of HCC–Fe3O4 was smooth and uneven (Fig. 1b). Irregular pores found from Fig. 1c proved that HCC–Fe3O4 obtained by the magnetic hydrothermal cross-linking method had different spatial organization from HCC. Pores in HCC–Fe3O4 provides necessary channel and adsorption space for the adsorption and ion diffusion of metal ions in solution. However, apparent differences in the surface morphologies of the adsorbent after uranyl ion adsorption were observed (Fig. 1d). Small particles of materials have gathered together. These results partially demonstrated that the chemical interactions took place between uranyl ion ions and the adsorbent.
The FT-IR patterns of HCC, HCC–Fe3O4 and uranium loaded HCC–Fe3O4 are shown in Fig. 2. For three pattems of HCC, HCC–Fe3O4 and uranium adsorbed HCC–Fe3O4, the bands around at 3400 cm−1 was associated with the −OH vibration or −NH3 vibration, and the bands around at 1612 cm−1 was denoted as the C=C vibration or C=O vibration, the broad band around 1303 cm−1 was relevant to −CH2 or −CH3 stretching vibrations. For the spectrum of HCC–Fe3O4 (Fig. 2b) the peak at 592 cm−1 was assigned to the Fe–O bond vibration of HCC–Fe3O4, compared with the spectrum of HCC, there was a new peak in the spectrum, proving the load of Fe3O4 on HCC. For the spectrum of HCC–Fe3O4-U (Fig. 2c) the adsorption peaks at 896 cm−1 was assigned to uranyl ion vibration, showing that uranium was adsorbed.
Figure 3 shows the XRD patterns of HCC–Fe3O4. It was XRD patterm overlay of iron oxide and chitosan. The d-spacing values of new significant peaks matched well with data in the JCPDS card (65-3107) for Fe3O4.
Figure 4 shows the magnetic properties of HCC–Fe3O4 (A) and HCC (B) at room temperature. The system of HCC–Fe3O4/water (A) and HCC/water (B) were firstly in the state of turbidity by drastically shake, followed by setting poles. After 10 min, it could be observed that HCC/water (D) was still cloudy while HCC–Fe3O4/water (C) was divisive and the solution is clear. Thus HCC–Fe3O4 dispersed in water solution could be easily separated from water with a magnet.
Effect of solution pH
pH is one of the important factors that affect the adsorption efficiency, because functional groups and ions species are affected. The pH effect on uranium(VI) adsorption can be explained by the surface characteristics of the adsorbents and the solute speciation. The effect of pH value on the adsorption of uranium from aqueous solutions is shown in Fig. 5. The adsorption of uranium(VI) gradually increased as pH increased from 3.0 to 7.0, and then decreased when pH was higher than 7.0. The adsorption of uranium on HCC and HCC–Fe3O4 composites was affected by pH through hydrolysis of uranyl ion in aqueous solution. At pH 3.0–6.0, uranium was hydrolyzed in forms of (UO2)2(OH) 2+2 or (UO2)3(OH) +5 (Fig. 6) species [19]. Moreover, more reactive sites were available with pH increase. As pH was higher than 7.0, the low adsorption affinity anion (UO2)3(OH) −7 increased, leading the decrease of uranium(VI) uptake. As a consequence, pH 7.0 was the optimal for uranium(VI) adsorption on HCC and HCC–Fe3O4.
Effect of contact time on the adsorption
The effect of contact time on adsorption of uranium(VI) on HCC and HCC–Fe3O4 was investigated to determine the equilibrium point and the result was given in Fig. 7. q for HCC and HCC–Fe3O4 increased rapidly resulting from surface adsorption in 0–25 and 0–75 min, later increased slowly through the internal diffusion and finally reached equilibrium at ca. 180 min.
Kinetics of the uranium(VI) adsorption consisted of three phases: an initial rapid phase, a slower second phase, and an equilibrium phase. The drastic uranium(VI) removal in the initial phase resulted from the instantaneous adsorption stage or external surface adsorption. The slower second phase was attributed to the intraparticle diffusion or the ion exchange in the inner surface of HCC and HCC–Fe3O4. At 25 °C, the adsorption of uranium(VI) reached equilibrium after 180 min. With the occupation of the active sites and the decrease of the uranium(VI) concentration, the uptake rate decreased until equilibrium. Figure 7 shows that uranyl adsorbed by HCC–Fe3O4 is faster than it by HCC. It might be due to the smooth and uneven surface of HCC–Fe3O4 and disordered fiber structure of HCC. However, HCC had a higher adsorption capacity than HCC–Fe3O4. Its adsorption sites had been occupied by Fe3O4, so this was a reasonable phenomenon.
To analyze the kinetic behaviors of uranium(VI) adsorption on HCC and HCC–Fe3O4, pseudo-first-order and pseudo-second-order models were used to fit the adsorption process [20]:
where k 1 (min−1) and k 2 (g mg−1 min−1) represent the kinetic rate constants of pseudo-first-order and pseudo-second-order models, respectively. q e (mg g−1) is the equilibrium adsorption amount, and q t (mg g−1) is the amount of uranium(VI) adsorbed at time t.
Plots of Ln(q e − q t ) to t and t/q t to t were fitted. The kinetic adsorption data were obtained and the results as shown in Fig. 8. From the linear plot of Ln(q e − q t ) versus t and t/q t versus t, k 1, k 2, and theoretical q e values (q e,cal) can be obtained. The calculated kinetic parameters from both models are shown in Table 1.
As could be seen, the correlation coefficient (R 2) of the pseudo-second-order model was higher than that of the pseudo-first-order model, and the q e,cal value for the pseudo-second-order model was more approximate to the experimental value (\(q_{e,\exp}\)). These results suggested that the pseudo-second-order model described the adsorption process well, implying that chemisorption or strong surface complexation controlled the adsorption of uranium(VI) on HCC and HCC–Fe3O4.
Effect of initial uranium(VI) concentration
The initial concentration are found to have strong effect on the adsorption and speciation of uranium(VI) in aqueous solutions. It could provide an important driving force to overcome all mass transfer resistance of uranium between the aqueous and solid phases [21]. The effect of the initial uranium(VI) concentration on the adsorption removal efficiency was studied by contacting a fixed mass of HCC and HCC–Fe3O4 (10 mg) at a fixed temperature (25 °C) and initial pH (7.0) using a range of initial uranium(VI) concentration (10, 20, 30, 50, 60, 70, 80 and 90 mg/L). The results are shown in Fig. 9. It was clear that the adsorption removal efficiency of uranium(VI) decreased with the initial uranium(VI) concentration increase in the aqueous solution. This attributed to the higher mobility of uranyl ion uranyl ion in the diluted solutions, resulting interaction of uranium(VI) and the adsorbent increase. The larger concentration gradient between adsorbents/solution interface caused the stronger equilibrium uptake [22].
Adsorption isotherms
Generally speaking, adsorption isotherms can provide some significant information for optimizing the application of adsorbents. Descriptions about the interaction between adsorption capacity and bond energy, adsorbents and sorbates, can be determined from isotherm models. The linear Langmuir isotherm is expressed as following:
where C e (mg L−1) is the equilibrium concentration of uranium(VI) in the liquid phase, q e (mg g−1) is the amount of uranium(VI) adsorbed on the solid phase, q max (mg g−1) represents the maximum adsorption amount and b is a constant related to the enthalpy of adsorption.
The Freundlich model is usually appropriate for heterogeneous adsorption in the following form and expressed as following:
where K F is the Freundlich constant related to the adsorption capacity and n is a constant representing the mutual interactions among adsorbed species.
Isotherm experimental data were simulated with Langmuir and Freundlich isotherm models. Isotherm parameters calculated from fitting processes are listed in Table 2. It could be seen that the Langmuir model fitted the isotherm data with a higher correlation coefficient (R 2) of 0.99 and 0.998, better than the Freundlich model. The Langmuir model indicated that uranium(VI) was monolayerly adsorbed on specific sites of HCC–Fe3O4. The maximum adsorption capacity of HCC–Fe3O4 was determined as 263.1 mg/g, while HCC was 404.5 mg/g. Moreover, HCC–Fe3O4 was much easier to be prepared than the chelator-integrated composite materials, and had more excellent acid-resistant properties with pH 3–7 (Fig. 10).
Adsorption thermodynamics
The effect of temperature on the adsorption of uranyl ion from aqueous solution (60 mg/L) under optimum conditions of pH and shaking time was studied. Thermodynamic parameters were computed using Van’t Hoff equation in form Eq. 7.
where K d is the equilibrium constant, ΔH°, ΔS°, ΔG°, and T are the enthalpy, entropy, Gibbs free energy, and temperature in Kelvin, respectively. R is the gas constant (8.314 J mol−1 K−1).
The ΔH° and ΔS° values were obtained from the slope and intercept of linear Van’t Hoff plot of ln K d versus 1/T (Fig. 11). The Gibbs free energy (ΔG) was calculated as
Values of thermodynamic parameters for the adsorption of uranium(VI) on HCC and HCC–Fe3O4 are given in Table 3. The negative values of ΔG at various temperatures indicated the spontaneous nature of the adsorption process. The decrease in ΔG with increasing temperature showed that the adsorption was less favorable at high temperature [23]. The positive value of ΔH indicated that the adsorption of uranium was endothermic. Similar results had been reported for the uranium(VI) adsorption [17, 24, 25]. The positive value of ΔS indicated that randomness increase at the solid-solution interface in the adsorption reaction [26] and that the influence of entropy was more remarkable than enthalpy of activation [27].
Selective adsorption
In order to investigate the adsorption selectivity of HCC and HCC–Fe3O4 for uranium, coexisting ions such Mg(II), Na(I), Zn(II), Mn(II), Co(II), Cu(II), Ni(II), Sr(II), Cs(I) and Hg(II) were added into the system at the optimal conditions. The results shown in Fig. 12 indicated that HCC showed no selectivity to uranium(VI). And the amount of uranium(VI) adsorbed on HCC–Fe3O4 (120.6 mg/g) was much higher than coexisting ions, demonstrating a better selectivity for uranium(VI).
Selectivity coefficient (\(S_{{\text{UO}}_{2}^{{2+}}}/M^{{\rm n} +}\)) for uranyl ions relative to coexisting ions was studied by Eq. 9.
where \(K_{d}^{{UO_{2}^{2 + } }}\) and \(K_{d}^{{M^{n + } }}\) are distribution coefficients of uranyl ion and other ion, respectively.
The relative selectivity coefficient S r is calculated according Eq. 10.
The resulting \(S_{{HCC - Fe_{3} O_{4} }}\), S HCC, K d , \(K_{d}^{{UO_{2}^{2 + } }}\) and S r were listed in Table 4. It was clear that values of K d and S of HCC–Fe3O4 was same to HCC. These results indicated that the selective properties of HCC toward uranium(VI) was not changed after grafting Fe3O4 groups.
Desorption and regeneration studies
The recycling and regeneration ability were significant for practical application of adsorbents. Adsorbents having excellent adsorption capacity as well as high desorption property will reduce secondary pollution and the overall cost. Thus the desorption experiment was performed to evaluate the recyclable availability of HCC–Fe3O4. Desorption experiments were performed using five kinds of eluents: 1.0 mol L−1 Na2CO3, 1.0 mol L−1 NaOH, 1.0 mol L−1 H2O, 1.0 mol L−1 H2SO4 and 1.0 mol L−1 EDTA. The desorption percentage for corresponding desorbing agents were 91.2, 75.6, 6.1, 8.2 and 54.7 % (Fig. 13), respectively. The maximum desorption percentage was found using Na2CO3. To assess reusability of HCC–Fe3O4, the adsorption–desorption experiment with 1.0 mol L−1 Na2CO3 was repeated for five cycles.
The results of five consecutive adsorptions and desorptions are shown in Fig. 14. The adsorption efficiency of HCC–Fe3O4 was still over 88 % after the fifth cycle. Meanwhile, after five cycles, HCC–Fe3O4 had a high magnetic sensitivity. The regeneration and reuse results showed that HCC–Fe3O4 was an effective and stable adsorbent for uranium(VI) removal.
Conclusions
In this study, HCC and HCC–Fe3O4 were used to remove uranium(VI) from aqueous solutions. The adsorption was found to be strongly dependent on pH. The uptake of uranium(VI) by HCC and HCC–Fe3O4 were maximal at pH 7. The kinetic studies showed that the pseudo-second-order model described uranium(VI) adsorption kinetics well. Thermodynamic parameters suggested that the adsorption of uranium(VI) on HCC and HCC–Fe3O4 were spontaneous and endothermic processes. Moreover, the magnetic HCC–Fe3O4 could be easily separated from aqueous solution with a magnet after adsorption. The results illustrated that HCC and HCC–Fe3O4 could be promising candidates as an adsorbent for uranium(VI) removal from aqueous solutions.
References
Shahandeh H, Hossner LR (2002) Role of soil properties in phytoaccumulation of uranium. Water Air Soil Pollut 141:165–180
Majdan M, Pikus S, Gajowiak A, Gładysz-Płaska A, Krzyżanowska H, Żuk J, Bujacka M (2010) Characterization of uranium(VI) sorption by organobentonite. Appl Surf Sci 256:5416–5421
Xie S, Yang J, Chen C, Zhang X, Wang Q, Zhang C (2008) Study on biosorption kinetics and thermodynamics of uranium by Citrobacter freudii. J Environ Radioact 99:126–133
Chen S, Hong J, Yang H, Yang J (2013) Adsorption of uranium(VI) from aqueous solution using a novel graphene oxide-activated carbon felt composite. J Environ Radioact 126:253–258
Augustine S, Gagnaire B, Adam-Guillermin C, Kooijman SA (2012) Effects of uranium on the metabolism of zebrafish, Danio rerio. Aquat Toxicol 118–119:9–26
Mudd GM (2008) Radon releases from Australian uranium mining and milling projects: assessing the UNSCEAR approach. J Environ Radioact 99:288–315
Li Y, Sun J, Du Q, Zhang L, Yang X, Wu S, Xia Y, Wang Z, Xia L, Cao A (2014) Mechanical and dye adsorption properties of graphene oxide/chitosan composite fibers prepared by wet spinning. Carbohydr Polym 102:755–761
Ziegler CJ, Sabin JR, Geier GR 3rd, Nemykin VN (2012) The first TDDFT and MCD studies of free base triarylcorroles: a closer look into solvent-dependent UV-visible absorption. Chem Commun 48:4743–4745
Xu J, Chen M, Zhang C, Yi Z (2013) Adsorption of uranium(VI) from aqueous solution by diethylenetriamine-functionalized magnetic chitosan. J Radioanal Nucl Chem 298:1375–1383
Shi Y, Zhang Q, Feng L, Xiong Q, Chen J (2014) Preparation and adsorption characters of Cu(II)-imprinted chitosan/attapulgite polymer. Korean J Chem Eng 31:821–827
Zeng M, Zhang T-A, Lv G-Z, Dou Z-H, Liu Y, Zhang Y (2014) Adsorption of Au(III) ions on xanthated crosslinked chitosan resin in hydrochloric acid medium. Rare Met 014:0279–0882
Yang Y, Wu WQ, Zhou HH, Huang ZY, Ye TT, Liu R, Kuang YF (2014) Adsorption behavior of cross-linked chitosan modified by graphene oxide for Cu(II) removal. J Cent South Univ 21:2826-2831
Yang X, Zhang X, Ma Y, Huang Y, Wang Y, Chen Y (2009) Superparamagnetic graphene oxide–Fe3O4 nanoparticles hybrid for controlled targeted drug carriers. J Mater Chem 19:2710
Tsang SC, Caps V, Paraskevas I, Chadwick D, Thompsett D (2004) Magnetically separable, carbon-supported nanocatalysts for the manufacture of fine chemicals. Angew Chem 43:5645–5649
Elliott DW, Zhang W-X (2001) Field assessment of nanoscale bimetallic particles for groundwater treatment. Environ Sci Technol 35:4922–4926
Šafaíková M, Šafaík I, (1999) Magnetic solid-phase extraction. J Magn Magn Mater 194:108–112
Zhang X, Wang J, Li R, Dai Q, Gao R, Liu Q, Zhang M (2013) Preparation of Fe3O4@C@layered double hydroxide composite for magnetic separation of uranium. Ind Eng Chem Res 52:10152–10159
Savvin SB (1961) Analytical use of arsenazo III Determination of thorium, zirconium, uranium and rare earth elements. Talanta 8:673–685
Wang G, Liu J, Wang X, Xie Z, Deng N (2009) Adsorption of uranium(VI) from aqueous solution onto cross-linked chitosan. J Hazard Mater 168:1053–1058
Wu Y, Luo H, Wang H, Wang C, Zhang J, Zhang Z (2013) Adsorption of hexavalent chromium from aqueous solutions by graphene modified with cetyltrimethylammonium bromide. J Colloid Interface Sci 394:183–191
Zou YD, Cao XH, Luo XP, Liu Y, Hua R, Liu YH, Zhang ZB (2015) Recycle of U(VI) from aqueous solution by situ phosphorylation mesoporous carbon. J Radioanal Nucl Chem 306:515–525
Anirudhan TS, Radhakrishnan PG (2009) Improved performance of a biomaterial-based cation exchanger for the adsorption of uranium(VI) from water and nuclear industry wastewater. J Environ Radioact 100:250–257
Fasfous II, Dawoud JN (2012) Uranium(VI) sorption by multiwalled carbon nanotubes from aqueous solution. Appl Surf Sci 259:433–440
Gao JK, Hou LA, Zhang GH, Gu P (2015) Facile functionalized of SBA-15 via a biomimetic coating and its application in efficient removal of uranium ions from aqueous solution. J Hazard Mater 286:325–333
Peng GW, Ding DX, Xiao FZ, Wang XL, Hun N, Wang YD, Dai YM, Cao Z (2014) Adsorption of uranium ions from aqueous solution by amine-group functionalized magnetic Fe3O4 nanoparticle. J Radioanal Nucl Chem 301:781–788
Zhang X, Jiao C, Wang J, Liu Q, Li R, Yang P, Zhang M (2012) Removal of uranium(VI) from aqueous solutions by magnetic Schiff base: kinetic and thermodynamic investigation. Chem Eng J 198–199:412–419
Anirudhan TS, Rijith S (2012) Synthesis and characterization of carboxyl terminated poly(methacrylic acid) grafted chitosan/bentonite composite and its application for the recovery of uranium(VI) from aqueous media. J Environ Radioact 106:8–19
Acknowledgments
This work was financially supported by the National Basic Research Program of China (No. 2014CB460604), the National Natural Science Foundation of China (Grant No. 21301028, 11475044, 41461070, 21561002), the Program for Changjiang Scholars and Innovative Research Team in University (Grant No. IRT13054), the Science & Technology Support Program of Jiangxi Province (Grant No. 20141BBG70001, 20151BBG70010), the Advanced Science & Technology Innovation Team Program of Jiangxi Province (Grant No. 20142BCB24006), the Innovation Team Program of Jiangxi Provincial Department of Science and Technology (Grant No. 2014BCB24006), and the Project of East China Institute of Technology Graduate Student Innovation Fund (Grant No. DHYC2014025, DHYC2015011).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yu, Sl., Dai, Y., Cao, Xh. et al. Adsorption of uranium(VI) from aqueous solution using a novel magnetic hydrothermal cross-linking chitosan. J Radioanal Nucl Chem 310, 651–660 (2016). https://doi.org/10.1007/s10967-016-4898-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-016-4898-y